All published articles of this journal are available on ScienceDirect.
A Detailed Insight of the Anti-inflammatory Effects of Curcumin with the Assessment of Parameters, Sources of ROS and Associated Mechanisms
Abstract
Background:
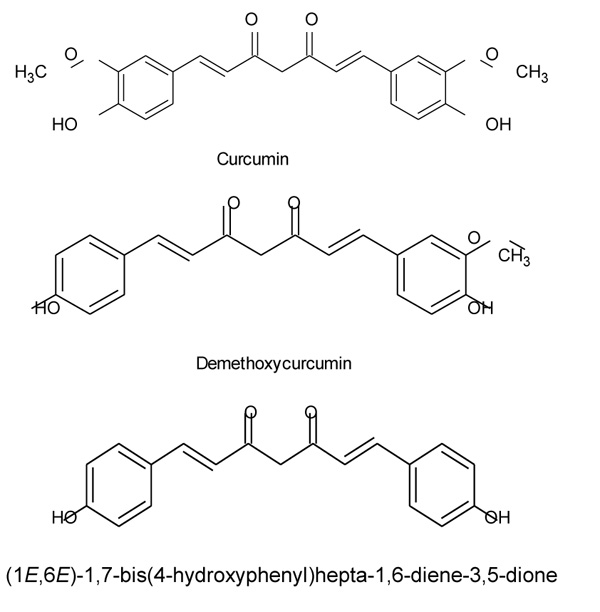
Curcumin is an active constituent of Curcuma longa, which belongs to Zingiberaceae family. It is derived from the Rhizome of a perennial plant having molecular formula C21H20O6 and chemically it is (1, 7- bis (4- hydroxy - 3 methoxyphenyl) -1, 6 - heptadine - 3, 5 - diene), also known as diferuloylmethane. Curcumin has been extensively used as a herbal constituent for curing several diseases and is scientifically proven to show major effects as an anti-inflammatory agent.
Objective:
Inflammation is an important factor for numerous diseases including diabetes neuropathy, cancer, asthma, arthritis, and other diseases. Prophylaxis of inflammatory diseases through synthetic medications tends to have major toxicity and side effects on a large number of population. The foremost aim of this review paper is to assess the natural anti-inflammatory effect of curcumin, source, and mechanism of action, potential therapeutic effect and models associated. Additionally, this paper aims to scrutinize inflammation, sources of reactive oxygen species, and pathways of reactive oxygen species generation and potential side effects of curcumin.
Methods:
Selection of data has been done by studying the combination of research and review papers from different databases like PubMed, Medline and Web of science from the year 1985- 2018 by using search keywords like “curcumin”, “anti-inflammatory”, “ROS”, “Curcuma longa”, “medicinal uses of curcumin”, “assessing parameters”, “inflammation”, “anti-oxidant”
Results:
On the basis of our interpretation, we have concluded that curcumin has potential therapeutic effects in different inflammatory diseases, it inhibits the inflammatory mediators, oxidation processes, and oxidative stress and has no severe toxicity on animals and humans.
Conclusion:
Oxidative stress is a major cause of inflammation and curcumin has a good potential for blocking it. Curcumin is also easily accessible herbal source and should be consumed in the form of food, antioxidant, anti-inflammatory agents and further observation should be done on its therapeutic parameters, risk factors, and toxicity studies and oral viability.
1. INTRODUCTION
Curcumin (Curcuma longa) is a spice which belongs to Zingiberaceae family. It is derived from the Rhizome of a perennial plant [1]. It is low in molecular weight and chemically it was initially characterized in 1910 with a molecular formula of C21H20O6 [2]. The remedial properties of this herb are known from ancient times. Chemically, curcumin is (1, 7 - bis (4 - hydroxy - 3 methoxyphenyl) - 1, 6 - heptadine - 3, 5 - diene), (Fig. 1) it is also known as diferuloylmethane as shown in (Fig. 1) Diferuloylmethane is one of the main polyphenyls found in this herb (Curcuma longa). In Asian Countries, Curcuma longa has extensively been used because it has a wide range of medicinal effect like antioxidant, anti-inflammatory [2], anti-mutagenic, anticancer and antimicrobial properties [3]. The active constituents which show medicinal property in this herb are curcuminoids present in Rhizome. So many studies have shown the pharmacokinetics of curcumin and proposed that it is poorly absorbed from the intestine. Studies have shown that oral absorption in Rats is poor approximately 75% of the constituents being excreted in the feces and in urine traces appeared while administration through i.p shows only 11% curcumin in bile, accounting poor absorption of curcumin through the intestine [4]. Curcumin acts as an anti-inflammatory by inhibiting cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (iNOs) and lipoxygenase (COX). INOs, LOX, and COX are key enzymes that mediate inflammatory processes. The inappropriate up-regulation of COX -2 and /or ins has found to be linked with the physiopathology of certain inflammatory disorders [2].
1.1. Inflammation
Historically, inflammation was characterized by some visual features like swelling, redness heat, pain, and loss of function, the former four features have been given by a Rome scientist Caelus. A short time ago, inflammation has been defined as “the series of transformations which happens in biological tissue after sudden laceration or trauma or abrasion if only the trauma or abrasion is not of such kind that at once it demolishes its morphology and endurance”. However, previously inflammation was perceived as being a stage in the process of curing, from the 19th century, inflammation was perceived as unwanted responses which can be destructive to the cell of the host [5]. In order to understand the anti-inflammatory effect curcumin, firstly there is a need to understand the involvement or role of inflammation in pathological and physiological procedures like wound healing and infection. In order to cure an injury, signal transduction initiates and encourage the host to heal the tissue. The Neutrophils, monocytes, and eosinophils from the venous, plays a major role in this mechanism. Neutrophils perform a four-step procedure and which are believed to support the enlistment of these cells which are inflammatory to the site of injured tissue and to extracellular matrix (ECM), the endothelium and fibroblast proliferate and move for the rebuilding of the normal environment. Following steps involved are activation of (L-P- and E- selectin); immobilization of neutrophils and transmigration [6].
2. MATERIALS AND METHODS
A literature search was made on databases like Pubmed and Medline by using the keyword Curcumin, Anti-inflammatory, ROS, inflammation and inflammasome. A combination of research and review paper was found and to get the most suitable article nonrelevant data were excluded. Selection of data has been done by studying the combination of research and review papers from different databases like PubMed, Core, Science3open, Directory of open access journals, EMBASE, Europe PMC, FSTA- Food Science and Technology, Nutrition, Google Scholar, HubMed, Merck Index, MedlinePluse, Indian citation index, Science Open, PubMed, Scopus, Semantic Scholar, World Wide Science, Shodhganga, Science Direct from year 1985- 2018 by using search keywords like curcumin , anti-inflammatory , ROS , curcuma longa , medicinal uses of curcumin , assessing parameters , inflammation , anti-oxidant.
2.1. Inclusion and Exclusion of Databases
500 publications were searched using keywords Curcumin, Anti-inflammatory , ROS , inflammation and inflammasome in databases PubMed, Core, Science3open, Directory of open access journals, EMBASE, Europe PMC, FSTA- Food Science and Technology, Nutrition, Google Scholar, HubMed, Merck Index, Medline Plus, Indian citation index, Science Open, PubMed, Scopus, Semantic Scholar, World Wide Science, Shodhganga, Science Direct from year 1985- 2018.
2.2. Inclusion
96 References were included because of the following reasons:
- Papers were relatable to the topic.
- Papers were published in the reputed journals.
- Papers were clear and were available in a suitable English language.
2.3. Exclusion
Rest papers were excluded because of the following reasons:
- Either papers were unclear or there were no proper conclusions.
- Few papers were not from the reputed journals and data was missing.
- Either explanatory images were not found in context to our topic or papers were not available in the English language.
2.4. Assessing Parameters Involved in Inflammation
ROS source and regulation: ROS have moderately reduced oxygen metabolites, possessing high oxidizing potential. In low concentrations, they assist convoluted signaling functions but are injurious at a high concentration because oxidize cellular content like lipid and protein resulting in DNA damaging. ROS acts as signaling molecules and modulate cell growth, apoptosis, and adherence to neighbor cells, differentiation, and infirmity [7].
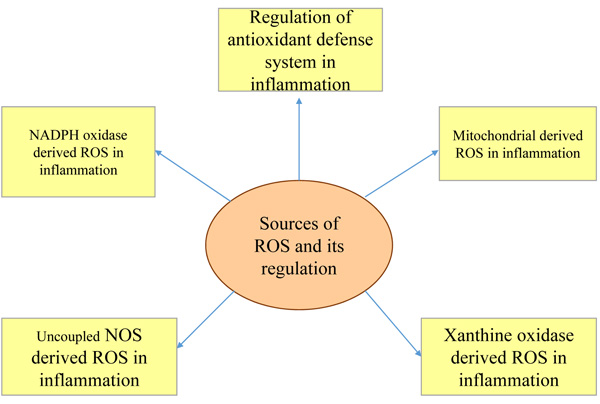
2.5. Sources of ROS and their Regulation
The major sources of ROS are Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase derived ROS in inflammation, mitochondria-derived Reactive Oxygen Species (ROS) in inflammation, xanthine oxidase derived Reactive Oxygen Species (ROS) in inflammation, and uncoupled NOS derived Reactive Oxygen Species (ROS) in inflammation and regulation of antioxidant defense system in inflammation (Fig. 2). Because of the prolonged generation of ROS, the chronic inflammatory disease progression takes place [8]. Superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radical (OH), and hypochlorous acid (HOCL) are the biologically relevant ROS as per studies. ROS are also generated as a byproduct in cellular metabolic reactions taking place in mitochondria through electron transport chain (etc) and cytochrome P450 [9].
2.6. ROS Production and Defense Mechanism in the Human Mitochondrial Cell
The production of superoxide occurs by uncoupled endothelial nitric oxide synthase (eNOS), Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase and xanthine oxidase derived molecular reduction of oxygen. Superoxide O2 undergoes dismutation and form H2O2 by superoxide dismutase (SOD). O2 also reacts with nitric oxide and form peroxynitrite (ONOO), this is four times faster than O2 dismutation of H2O2. Further, highly reactive HOCL can form at inflammatory sites in the presence of Myeloperoxidase (MPO), this is indicated in neutrophils in ample amount. In the presence of fe+2, H2O2 can also form OH which is highly toxic this reaction is called Fenton's reaction. This reaction is catalyzed by peroxiredoxins (prx) or glutathione peroxidase (GPX). To detoxify H2O2 Prx uses thioredoxin (Trx) (Fig. 3) [7]. The summary of the whole process can be given by Haber-weiss reaction and antioxidant reactions [9].
Antioxidants Reaction
2O2 + 2H+ H2O2 + O2
2H2O2 H2O + O2
H2O2 + 2 GSH 2H2O + GSSG
H2O2 + Prx (SH) 2 2H2O + PrxSS
PrxSS + Trx (SH) 2 Prx (SH) 2 + TrxSS
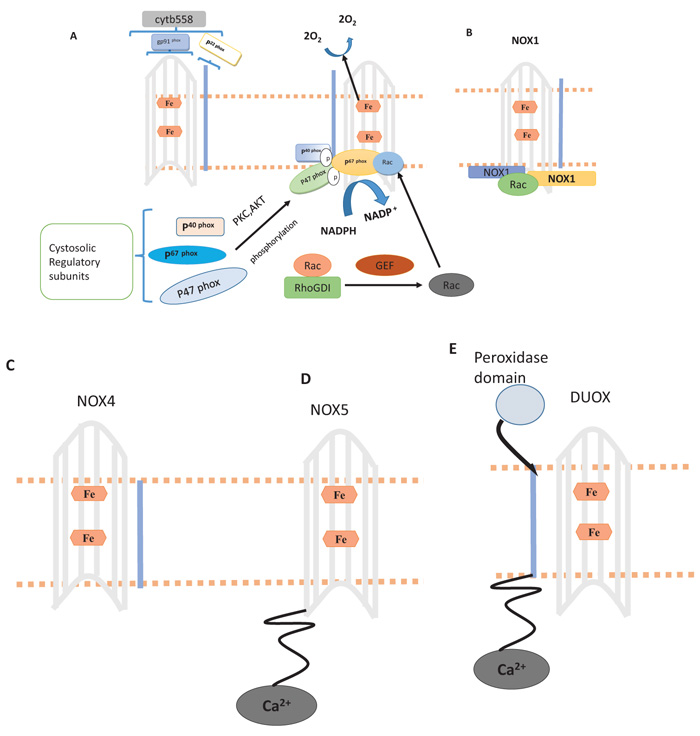
2.7. Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase
NADPH oxidases, firstly recognized while inducing respiratory burst and bacterial killing in phagocytes [10] There are homologs of NADPH oxidase (NOX1-NOX5, Duox and 2), they are dissimilar in structures, their mechanism in different tissues [11]. The NADPH oxidase structure is defined in the Fig. (4). These homologs play a major role in vascular inflammation [7]. For activation of gp91phox two self-supporting events are needed, results in the association of regulatory proteins, present in the cytosol (p67 phox, p22 phox, and p40phox) along with flavocytochrome b558. The former event causes activation of Protein Kinase C (PKC) and AKT, resulting in phosphorylation of autoinhibitory zone (AIR) of p47 phox, hence violating inhibition through the autoinhibitory loop and allowing p47 phox to attach with p22 phox. During second step GDP replacement with GTP occurs through (RhoGDI), encouraging its attachment with p67 phox, resulting in active complex formation. The hexagon in figure A indicates heme, NOX1, NOX4 and NOX5 in B to E, are NADPH oxidase homologs in which NOX1 to NOX4 have identical gp91 phox core, NOX5 has intracellular N- terminal calcium binding domain, Duox1 is also constructed at NOX5 structure of gp91 phox. NOX5 carries alpha-helical domain on N- terminal membrane. Through the assembly of Rac, NOXA1 and NOXO1 ROS generation occur by NOX1. There is no need for cytosolic subunit for production of ROS by NOX4 instead require p22 phox. ROS production through NOX5 and DUOX occur by calcium and p22 phox subunit is not required [12-24].
2.8. Mitochondrial-derived Reactive Oxygen Species in Inflammation
An electron series forms a spatial arrangement in increasing order of their redox potential and get organized into four forms of complexes during mitochondrial etc. Electron transfer takes place between flavins (FMN H2 and FADH2) is shown by the arrows in those regions where complexes (I-IV) were formed, electron transfer also takes place between coenzyme Q (Q-QH2), iron-sulfur center (Fe-S), cytochrome (ca, a3 and c1) and molecular oxygen, all of these events lead to the formation of H2O Fig. (5). The major sites of ROS generation are two complexes, complex I and complex II, these complexes lead to the generation of reactive oxygen species [25-33].
2.9. Activation of Inflammasome Through Reactive Oxygen Species (ROS)
Raised reactive oxygen species generation within the cell either through mitochondrial electron chain transport or NADPH oxidase is recognized by Thioredoxin-Interacting Pgrotein (TXNIP) and Trx complex, which directs the binding of NLRP3 with TXINP, followed by NLRP3 activation and association of Asc and procaspase 1/12 proteins resulting in the construction of inflammasome (Fig. 6). Due to active NLRP3 inflammasome cleavage of interleukin-1β (IL-1) and pro-IL-18 to active IL-1β and IL 18, released by inflammatory cells, DAMP stands for danger associated molecular pattern; PAMP for the pathogen-associated molecular pattern; ASC stands for apoptosis-associated speck like protein [34-38].


2.10. Uncoupled NOS- derived ROS in Inflammation
eNOS enzyme has a homodimeric structure, it comprises of an oxygenase domain and a reductase. There is the binding site at eNOS interface for tetrahydrobiopterin (BH4) which is a co-factor along with Larginine. Through monoxygenation, eNOS form L-citrulline by L-arginine and NO (by-product). During this process of reaction, oxidation of BH4 occurs and which forms tri hydro pterin radicals which further get protonated at the position N5 in the form of BH3H+. (Fig. 7).due to an elevation of peroxynitrite level an oxidative stress results and which further causes oxidation gives BH2(biologically inactive) by BH4. BH2 cannot be taken back by the cell hence recycling of BH2 molecule gets interrupted and this leads to an uncoupled state of enzymes, reduction of oxygen to superoxide, block synthesis of NO [39-47].
2.11. Mode of Action of Curcumin as an Anti-inflammatory Agent
Oxidative stress is associated with many chronic diseases and its pathological conditions are firmly linked to inflammation, both of them are induced by one another. Inflammatory cells are also known to liberate numerous reactive species at its site which leads to oxidative stress. This manifests a link between inflammation and oxidative stress [48]. A number of reactive species can commence an intracellular signaling cascade that augments pro-inflammatory expression of genes. [49-51]. In so many chronic diseases like asthma, allergy, cancer, metabolic syndrome, multiple sclerosis, epilepsy, cardiovascular diseases, Parkinson's disease, cerebral injury, arthritis, psoriasis, diabetes, depression, obesity and Acquired Immune Deficiency Syndrome (AIDS) inflammation has been pointed out in the stage of all these disease progression [48]. The major inflammation mediator in various diseases is α (TNF-α) tumor necrosis factor and it is regulated by nuclear factor (NF)-κB. Among NF-κB activator, TNF-α is the most influential and regulation of its expression is also done by NF-κB. Most of the cytokines stimulate NF-κB, TNF-α, disease-causing viruses; mechanical, chemical, physical, mental stress; curcumin blocks NF-κB activation by following different stimuli of inflammation, curcumin has been shown to subdue inflammation via separate mechanisms [48].
3. CURCUMIN AS A POTENTIAL CURE FOR OTHER DISEASES
3.1. Neurodegenerative Diseases
With aging, human brain tends to accumulate different metal ions like copper (Cu), iron (Fe) and zinc (Zn). The human brain, however, has ample antioxidants that block the formation of ROS through Fenton reaction which involves metal ions reduction, boosting molecular oxygen [52]. As per curative point of view curcumin as a herbal source has been majorly shown to manifest antioxidant against major class of neurodegenerative diseases like neuropathic pain [53], Spongiform encephalopathies (Creutzfeld Jakob disease) [54], epilepsy [55], age-associated neurodegeneration [56], multiple sclerosis [57], cerebral injury [58], schizophrenia [59], Parkinson's disease [60], and depression [61]. Scientist Kim et al. (2001) found during his research that curcumin and few of its similar components like bisdemethoxycurcumin (BDMC) and demethoxycurcumin (DMC) reduce ROS generation in PC12 rat induced from Abeta- induced oxidative stress, and compounds were proved to show better antioxidant activity in comparison to alpha-tocopherol [62].
3.2. Cardiovascular Diseases
Through various reports, it has been found that inflammation contributes majorly to cardiovascular diseases [63]. One of which suggests that atherosclerosis is a result of damage of lipoproteins through oxidation i.e result of oxidative stress also damages blood vessels and membranes [64]. Second protolytic damage of one of elastin called median elastin is the cause of disease abdominal aortic aneurysms [65]. Many studies have concluded that curcumin protects the heart from cardiovascular disease. Srivastava et al. (1985) performed an experiment on animal to analyze the result of curcumin by inducing myocardial ischemia through ligation of left coronary artery, the heart was separated four hours earlier before the experiment and examined for myeloperoxidase(MPO), glutathione (GSH), superoxide dismutase (SOD), lactate dehydrogenase (LDH), catalase (CAT). They found after the study that curcumin helps to maintain blood pressure, control ischemia and protect the heart from cardiovascular diseases [66].
3.3. Diabetes
Hyperglycemia is a disease which majorly affects kidney, liver, brain, heart and other body organs. Inflammation plays an important role in the pathogenesis of diabetes [67]. Curcumin can control the blood sugar levels, enhance an antioxidant property of pancreatic β cells and also promotes stimulation of PPAR-γ [68].
3.4. Potential Therapeutic Effects of Curcumin in other Forms of Diseases
Kobayashi et al. (1997) performed an experiment and examined the potential effect of curcumin on interleukins especially IL-5 and IL-2, IL-4 through lymphocytes in case of asthma caused by dust mites and others factors like (GM-CSF). He found that curcumin inhibited the production of (GM-CSF), IL-4, IL-5, and IL2 production, hence concluded that curcumin can suppress allergic responses by stimulating eosinophil and cytokines production [69]. Curcumin has been also found to be effective in various other diseases like renal ischemia [70], Scleroderma [71], Psoriasis [72], Acquired immunodeficiency disease (AIDS) [73], Rheumatoid Arthritis (RA) and other arthritis diseases [74] and cancer [75].


4. MODELS ASSOCIATED WITH ANTI-INFLAMMATORY PROPERTIES OF CURCUMIN
4.1. Edema and Inflammation
Srimal et al. performed an experiment for edema and inflammation in mice by inducing carrageen. He found that at 50-200 mg/kg doses, curcumin shows the anti-inflammatory effect by reducing edema. With dose at 48mg/kg, about 50% anti-inflammatory effect was observed which is nearly equal to the effect of phenylbutazone and cortisone at the same dose [76].
4.2. Ulcerative Colitis
Studies have shown that curcumin suppresses mucosal damage in mice which was induced with colitis and at a regimen of 50mg/kg of curcumin before 10 days of 1,4,6- trinitrobenzene sulphonic acid induction, indicated better colonic structure and reduction in neutrophil infiltration was observed, as well as inhibition in peroxidation of tissue and suppression of inflammation was observed [77].
4.3. Rheumatoid Arthritis
Wistar female rat was induced with the streptococcal cell for arthritis, a dose of 4 mg/kg per day and for continuous four days was given before inducing arthritis, as a result blocked the inflammation in joints in acute phase up to 75% and in chronic phase up to 68% [78].
4.4. Pancreatitis
Curcumin has been shown to reduce stimulation of NF-κB and blocking induction of mRNA of TNF-α, interleukin 6 and iNOS, all these events take place in the pancreatic cell. It was found that in case one where pancreatitis was induced by cerulein and in the second case where pancreatitis was induced by ethanol, curcumin inhibited the inflammatory mediators, hence decreased the severity of the disease [79].
4.5. The Potential Side Effect of Curcumin and Oral Bioavailability Enhancement
According to FDA, curcumin is safe. Curcumin manifests many pharmacological effects but still, it is safe in humans and animals. Some studies were performed for the toxicity test of curcumin [80]. NTP has performed acute and chronic toxicity test in B6C3F1 mice and F344/N rats by using turmeric oleoresin at different doses and concluded that after observation, it was found that none of the animals died and no lesions and body toxicity was observed, only certain stains on fur and facial color alteration was found, no severe toxicity was pointed out [81]. A number of experimental reports have been showing the positive effect of curcumin along with chemotherapy [82-85]. The trials on human and animal study specifying the nontoxic and anti-inflammatory effect of curcumin have been summarized in Table 2 [91-95]. To improve oral bioavailability of curcumin, various chemical and technological methods are suggested till now. Microencapsulation which is polymer based has been found to be effective technique for the improvement of oral bioavailability of curcumin.
In vitro and ex vivo study has shown that there is good adhesive property in microparticles and because of this property oral bioavailability of curcumin has been found to be elevated [96].
5. RESULTS
On the basis of our interpretation, we have concluded that curcumin has potential therapeutic effects in different inflammatory diseases, it inhibits the inflammatory mediators, oxidation processes, oxidative stress and has no severe toxicity on animals and humans.
At normal physiology inflammation is the segment of the biological reaction of body cell or tissues towards dangerous or unwanted stimuli but at abnormal condition it leads to many severe diseases like pulmonary fibrosis, asthma, cancer, arthritis, diabetic neuropathy, inflammatory bowel disease and others. We have summarized scientific studies specifying Assessing parameters and inflammatory mediators associated with various inflammatory diseases in Table 1 [86-90].
5. DISCUSSION
There is a strong need to perform more studies on the therapeutic effect of curcumin in inflammatory diseases and as through studies, it was concluded that oral viability of curcumin is not so good, more experiments are required to enhance its viability through gut flora. During literature search we have found that polymer-based microencapsulation is useful in improving oral bioavailability of curcumin. We have reviewed a number of articles which explains the high therapeutic value and very low toxicity of curcumin in fatal diseases. Many researches are still in processes.
| Scientist Reported Data |
Inflammatory Mediators and Assessing Parameters |
Associated Diseases | Study Conducted on Animal / Cell Culture | Study Conducted on Human | Inference | |
|---|---|---|---|---|---|---|
| Duration of Study (Year) | Sample Size | |||||
| Nadrowski et al.(2010) [86] | Interleukin 6 (IL 6) | Atherosclerosis | NA | 2007- 2011 | 4979 | For systematic inflammation IL-6 is important biomarker and is responsible for atherosclerosis. |
| Henry et al.(2008) [87] | Interleukin -6 (IL- 6) | Depression | BV-2 microglia and Male BALB/c mice |
NA | NA | Decrease levels of IL-6 in blood cause behavioral changes. |
| Zhang et al. (2014) [88] | NLRP3 inflammasome | Depression | Male BALB/c mice | NA | NA | NLRP3 inflammasome is responsible for depressive behaviors. |
| Grivennikov et al. (2009) [89] | Interleukin -6 (IL- 6) | Lung cancer | Stat3F/F mice | NA | NA | Destruction of IL-6 trans-signaling delays growth of tumor. |
| Lin et al.(2017) [90] | Interleukin 6(IL 6) | Hepatocellular carcinoma | Rag1−/− mice And Rarres2−/− mice |
Elevated level of IL-6 leads to progression of lung cancer. | ||
| Scientist Conducted Study | Part of Curcuma Longa Plant Used |
Study Conducted on (Animal) |
Study Conducted on Human |
Trial Phase |
Models | Type of Study | Conclusion | Inference |
|---|---|---|---|---|---|---|---|---|
| Sample size | ||||||||
| Ashish Shubhas et al.(2013) [91] | Curcuminoids and oil free aqueous extract of C. longa (COFAE) |
Albino swiss Mice and Albino Wistar rats | NA | NA | Xylene induced ear edema and cotton pallet granuloma model | Comparative. | COFAE at dose level (p< 0.05) reduce inflammation in each animal model. On comparative study the oil free aqueous extract showed better anti-inflammatory effect as compared to curcuminoids. | The active constituents of curcumin has potent anti-inflammatory effect in its different forms. |
| Chang et al. (2001) [92] |
8000 mg curcumin Per day |
NA | 25 subjects | Phase I | NA | NA | The study showed that curcumin is not toxic to humans when administered for 3 months up to the dose of 8,000 mg/day (orally). | Curcumin is nontoxic to human. |
| Satoskar et al.(1986) [93] | Curcumin (1200 mg/d) and phenylbutazone (300mg/d) | NA | 45 subjects | NA | NA | Comparative Controlled trail. |
Controlled trial study was conducted (5-days) with 45 postsurgical patients divided into groups placebo, curcumin (1200 mg/d), and phenylbutazone (300 mg/d). Phenylbutazone and curcumin showed better anti-inflammatory effect. |
Curcumin showed better anti-inflammatory effect. |
| Bundy Rafe et al. (2004) [94] | 1 tablet of curcumin extract per 8th day | NA | 500 IBS (selected) 207 subjects (screened for study) |
Phase II | NA | Partially blinded, randomized, two-dose, pilot study. | The prevalence rate of inflammatory bowel syndrome found to be declined up to 41% and 57% and further the percentage declined to 53%-60% between base line after treatment in both groups. | The anti-inflammatory effect of curcumin has been seen in inflammatory bowel syndrome. |
| Chandran Binu et al. (2012) [95] |
Curcumin (500 mg) and Diclofenac Sodium (50 mg) | NA | 45 | Phase II | NA | Randomized, single-blinded, pilot study. | Patients were randomly allocated in 1:1:1 ratio for administration of curcumin 500 mg (Group I) or curcumin 500 mg + diclofenac sodium 50 mg (Group II), or diclofenac sodium 50 mg (Group III) over a period of 8 weeks. | Curcumin showed better anti-inflammatory effect and reduced pain also. |
CONCLUSION
Curcumin (Curcuma longa) belongs to Zingiberaceae family, is a perennial plant, having curcuminoids as the major active constituent. It exhibits a wide range of therapeutic property like anti-inflammatory, antioxidants, antimutagenic, anticancer and antimicrobial property.
Curcumin acts as an anti-inflammatory by inhibiting cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (ins) and lipoxygenase (COX). INOs, LOX, and COX are key enzymes that mediate inflammatory processes. The inappropriate up-regulation of COX -2 and /or ins has found to be linked with the physiopathology of certain inflammatory diseases. Therefore, consumption of curcumin in daily life can protect from inflammation and oxidation induced processes and can contribute to a healthy lifestyle.
Most of the studies have found that cucumin has the potential for curing inflammatory diseases as it blocks the mechanism of reactive oxygen species generation via inhibiting oxidative stress. Curcumin has prominent effect on inflammatory mediators like cytokines as a result of which it blocks the oxidation process in mitochondria of the cell and reduces inflammation.
Curcumin should be considered for formulations. It has no severe toxicity as per studies, no carcinogenesis lesions were found during toxicity studies, although it has the potential to act as anticancer agent in various types of cancer induced through inflammation. The mechanisms of actions and its anti-inflammatory parameters must be thoroughly evaluated for future researches to design new formulations for treatment of various life-threatening diseases.
LIST OF ABBREVIATIONS
| Cox | = Cyclooxygenase |
| Lox | = Lipoxygenase |
| eNOs | = endothelial Nitric Oxide synthase |
| FDA | = Food and Drug Administration |
| TNF-α | = Tumor Necrosis Factor |
| NF-κ B | = nNclear Factor Kappa-light- chain- enhancer of activated B cells |
| mRNA | = messenger RNA (ribonucleic acid) |
| IL | = interleukins |
| PPAR-γ | = Peroxisome Proliferator Activated Receptor gamma |
| ROS | = Reactive Oxygen Species |
| TXINP | = Thioredoxin Interacting Protein |
| FADH2 | = Flavin Adenine Dinucleotide |
| FMN | = Flavin Mononucleotide |
| NOX | = Mono Nitrogen Oxidase |
| AKT | = serine/threonine- specific protein kinase |
| Rho GDI | = RHO protein GDP dissociation inhibitors |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We are genuinely thankful to the department of Pharmacy, Pranveer Singh Institute of Technology, Kanpur, Uttar Pradesh, India, for immensely guiding us and helping while writing this review article.


