Value of the A2DS2 Score Combined with the Neutrophil-to-lymphocyte Ratio in Predicting Acute Ischemic Stroke-associated Pneumonia
Abstract
Objective:
We aimed to explore the risk factors for acute ischemic stroke-associated pneumonia (SAP) and evaluate the predictive value of the Age, Atrial fibrillation, Dysphagia, Sex, Stroke Severity (A2DS2) score, neutrophil-to-lymphocyte ratio (NLR), and a combination of both indices for acute ischemic SAP.
Methods:
Overall, 1,505 patients with acute ischemic stroke (AIS) were enrolled and divided into SAP and non-SAP groups. Patients’ age, sex, and medical history (alcohol consumption, hypertension, diabetes, hyperlipidemia, coronary disease, atrial fibrillation, chronic obstructive pulmonary disease, and stroke history) were recorded. Clinical data were recorded, including consciousness disturbance, dysphagia, indwelling nasogastric tube, thrombolytic therapy, hospital stay length, National Institute of Health Stroke Scale (NIHSS) score, stroke position, TOAST classification, and blood pressure on admission. Laboratory indicators, including white blood cell (WBC) count, neutrophil count, lymphocyte count, creatinine, homocysteine, and fasting blood glucose, were also recorded. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. All patients were scored using A2DS2. Binary logistic regression was used to analyze the relationships between A2DS2, NLR, and SAP. Receiver operating characteristic (ROC) curves were generated to evaluate the diagnostic value of A2DS2, NLR, and their combined indices for predicting SAP.
Results:
SAP occurred in 203 (13.5%) of the 1,505 enrolled patients. Patients in the SAP group were older and had a higher proportion of hypertension and chronic obstructive pulmonary disease history, consciousness disorder, dysphagia, indwelling nasogastric tube, fasting blood glucose level, NIHSS score, and longer hospital stay. The SAP group had a higher A2DS2 score than the non-SAP group. Similarly, the WBC count, neutrophil count, and NLR were significantly higher in the SAP group than in the non-SAP group. After excluding confounding factors, binary logistic regression analysis showed that age, NIHSS score, NLR, and A2DS2 score were independent risk factors for SAP. The ROC curves showed the A2DS2 score and NLR predicted SAP with an area under the curve (AUC) of 0.855 (sensitivity: 73.3%, specificity: 86.1%) and 0.849 (sensitivity: 79.7%, specificity: 80.6%), respectively, and the combined prediction of SAP AUC was 0.924 (sensitivity: 87.7%, specificity: 82.8%), which was higher than that of a single index, with improved the sensitivity of prediction.
Conclusion:
In patients with AIS, the A2DS2 score combined with NLR is of greater value in predicting the risk of acute ischemic SAP than a single indicator.
1. INTRODUCTION
Acute ischemic stroke (AIS) is the most common type of stroke, accounting for 69.6~70.8% of stroke cases in China [1]. It is characterized by high morbidity, disability, and mortality. After the stroke, patients are also prone to heart, lung, kidney, and other dysfunctions. Stroke-associated pneumonia (SAP) is a common complication in patients with stroke. It is defined as pneumonia in patients with stroke and non-mechanical ventilation within 7 days of onset. According to the Chinese National Stroke Registry, SAP incidence in patients with ischemic stroke was 11.4% [2]. It increases hospital stay and medical costs and leads to poor prognosis and increased disability and mortality rates [3, 4]. SAP is associated with multiple risk factors, including age, stroke severity, stroke site and type, dysphagia, consciousness disturbance, long-term bed stay, atrial fibrillation, chronic obstructive pulmonary disease (COPD), etc. [5].
Currently, there are multiple clinical scoring scales for early SAP prediction, such as the Age, Atrial fibrillation, Dysphagia, Sex, Stroke Severity (A2DS2) score, Acute Ischaemic Stroke-Associated Pneumonia Score(AIS APS), and the Prestroke Independence, Sex, Age, National Institutes of Health Stroke Scale(ISAN score), etc. [6-8]. Different scoring scales have different advantages and disadvantages, among which the A2DS2 score proposed by Hoffmann is the most widely used in clinical practice (Table 1) [8]. It is easy to perform and has a high predictive value for SAP [7-9]. However, the A2DS2 score only combines the patient's age and clinical manifestations and lacks the support of blood indicators, thus limiting its use.
| Assignment Project | Assignment Score |
|---|---|
| Age ≥75 | 1 |
| Atrial fibrillation | 1 |
| Male patients | 1 |
| Dysphagia | 2 |
| Initial NIHSS | - |
| 0~4 | 0 |
| 5~15 | 3 |
| ≥16 | 5 |
The neutrophil-to-lymphocyte ratio (NLR) is a routine blood test and a readily available and affordable indicator of systemic inflammation, with better predictions for bacterial infections than traditional inflammatory markers [10, 11]. The NLR can also predict the progression of post-stroke complications (including infection, depression, bleeding transformation, and early neurological deterioration) [12]. SAP is the most common infection after stroke, and NLR is closely related to SAP occurrence and development. Recent research has shown that the NLR is an independent predictor of SAP development in patients with AIS; a high NLR is associated with more severe pneumonia [13, 14], and a higher NLR 24 hours after AIS is associated with adverse stroke outcomes [15].
We aimed to evaluate the predictive value of the A2DS2 score combined with the NLR for SAP to provide a new method for the early screening of high-risk patients with SAP.
2. MATERIALS AND METHODS
2.1. Study Participants
Patients with AIS who were hospitalized in the Neurology Department of Shenzhen Second People's Hospital from January 2015 to December 2019 were recruited.
The inclusion criteria were as follows: 1) AIS diagnosis was consistent with the 2014 Chinese guidelines for the diagnosis and treatment of Acute Ischemic Stroke [16]. 2) AIS was confirmed by computed tomography (CT) or magnetic resonance imaging. 3) hospitalization within 48 hours of symptom onset. The exclusion criteria were: 1) intracranial or other systemic malignancies, 2) long-term use of immunosuppressive agents or cortisol hormones, 3) AIS complicated by severe liver, kidney, and cardiopulmonary insufficiency, 4) AIS combined with infectious diseases prior to admission, 5) presence of autoimmune and hematological diseases, and 6) death or discharge within 48 hours of admission.
2.2. Study Groups
Patients were divided into SAP and non-SAP groups. The diagnostic criteria for SAP were as follows [17]: First, at least one of the following was considered: 1) fever (body temperature ≥38°C) without other clear causes, 2) leukopenia (≤4×109/L) or leukopenia (≥10×109/L), and 3) age ≥70 years, and no other clear cause of the altered state of consciousness. Second, patients met at least two of the following criteria: 1) new purulent sputum, changes in sputum characteristics, increased respiratory secretion, or increased sputum aspiration times within 24 hours; 2) new or worsening cough, dyspnea, or shortness of breath (respiratory rate >25 times/min); 3) pulmonary auscultation revealed rales, crackles, or bronchial breathing sounds; 4) gas exchange disorders [such as hypoxemia (Pa02/Fi02 ≤240), increased oxygen demand]. In addition, chest X-ray or chest CT showed a new or progressive infiltrating shadow, consolidation shadow, or ground glass shadow.
We also divided the 1,505 patients into low- and high-NLR groups according to the median NLR.
2.3. Clinical Data
We collected data on age, sex, past medical history (alcohol consumption, hypertension, diabetes, hyperlipidemia, coronary disease, atrial fibrillation, COPD, and stroke history), consciousness disturbance, dysphagia, indwelling nasogastric tube, National Institute of Health Stroke Scale (NIHSS) score, whether thrombolysis was performed, length of hospital stay, stroke site and Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification, blood pressure on admission, and pneumonia occurrence within 7 days of stroke onset. Fasting peripheral blood results, including white blood cell (WBC) count, neutrophil count, lymphocyte count, creatinine, homocysteine, fasting blood glucose level, and other biochemical indicators, were obtained on the morning of the second day after admission. The scoring details for A2DS2 are presented in Table 1.
2.4. Statistical Analysis
Statistical analyses were performed using SPSS Statistics (version 26.0; IBM Inc., Armonk, NY, USA). Normally distributed data were represented as the mean±standard deviation, using a group t-test for comparison between two groups. Non-normally distributed data were represented as the interquartile range (P25, P75) using the Mann–Whitney U test. Quantitative data were expressed as percentages using the χ2 test or Fisher's exact probability method. Binary logistic regression analysis was used to assess independent risk factors for SAP. Clinical characteristics were compared between the different NLR groups using univariate analysis. Receiver operating characteristic (ROC) curves were used to assess the predictive value of the A2DS2 score, NLR, and SAP combination. The inspection level (α) was set to 0.05.
3. RESULTS
3.1. Comparison of Clinical Features between the SAP and Non-SAP Groups
A total of 1,505 eligible patients (mean age, 64 years; 65.8% men) were included, with a median NLR of 2.43 (1.74–3.53). There were 203 and 1,302 patients in the SAP and non-SAP groups, respectively. In this study, SAP incidence was 13.5%. Univariate analysis of the clinical data of the two groups showed that patients in the SAP group were older, more complicated with hypertension and COPD, with more consciousness disorders and dysphagia, requiring a higher proportion of gastric tube retention, with higher fasting blood glucose levels, and higher NIHSS scores. WBC and neutrophil counts were significantly higher in the SAP group than in the non-SAP group, whereas the lymphocyte count was lower in the SAP group than in the non-SAP group. The A2DS2 and NLR scores were significantly higher in the SAP group than in the non-SAP group (NLR: 5.46 [3.64-8.52] versus 2.24 [1.65-3.11]; P=0.000) and A2DS2 score: 5 [3-6] versus 1 [1, 2]; P=0.000). Also, the length of hospital stay was significantly longer in the SAP group than in the non-SAP group. However, there were no significant differences in smoking and drinking history, diabetes, hyperlipidemia, coronary disease, atrial fibrillation, stroke history, thrombolytic therapy, stroke site and type, blood pressure, creatinine, or homocysteine levels between both groups (P>0.05) (Table 2).
Factors with P<0.1 in the univariate analysis were considered independent variables, and whether SAP occurred was considered the dependent variable. A multivariate logistic regression analysis was conducted, and after adjusting for confounding factors, the results showed that age, NIHSS score, NLR, and A2DS2 score were the independent risk factors for SAP (Table 3).
3.2. Comparison of Clinical Features between the Low NLR (≤2.43) and High NLR (>2.43) Groups
SAP incidence was significantly higher in the high NLR group than in the low NLR group (24.2% versus 2.8%, P<0.001). Also, patients in the high NLR group were older, had more patients with a previous history of hypertension and cerebral hemorrhage, and had a higher proportion of patients with dysphagia and consciousness disturbance. Indwelling gastric tube use, systolic blood pressure on admission, WBC count, neutrophil count, lymphocyte count, creatinine and homocysteine levels, and NIHSS scores were higher in the high NLR group than those in the low NLR group. Furthermore, patients with a higher NLR stayed hospitalized for significantly longer, and their A2DS2 scores were higher than those of the lower NLR group. No significant differences were observed for the other parameters between both groups (P>0.05) (Table 4).
| - | SAP (n= 203) | Non-SAP (n=1302) | Z/χ2 | P-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 73 (61-83) | 63 (54-72) | -7.396 | 0 |
| Sex, male, n (%) | 123 (60.6%) | 868 (70.6%) | 6.246 | 0.1 |
| Stroke history, n (%) | 68 (33.5%) | 526 (40.4%) | 3.532 | 0.06 |
| alcohol drinking, n (%) | 47 (23.2%) | 331 (25.4%) | 0.481 | 0.488 |
| Hypertension, n (%) | 146 (71.9%) | 827 (63.5%) | 5.427 | 0.02 |
| Diabetes, n (%) | 69 (34.0%) | 349 (26.8%) | 4.725 | 0.094 |
| Hyperlipidemia, n (%) | 12 (5.9%) | 107 (8.2%) | 1.289 | 0.256 |
| Coronary disease, n (%) | 28 (13.8%) | 115 (8.8%) | 0.243 | 0.622 |
| Atrial fibrillation, n (%) | 33 (16.3%) | 68 (5.2%) | 0.483 | 0.487 |
| COPD, n (%) | 11 (5.4%) | 19 (1.5%) | 12.139 | 0 |
| Cerebral infarction, n (%) | 48 (23.6%) | 249 (19.1%) | 3.315 | 0.069 |
| Hematencephalon, n (%) | 8 (3.9%) | 41 (3.1%) | 0.350 | 0.554 |
| Disturbance of consciousness, n (%) | 43 (21.2%) | 52 (4.0%) | 87.735 | 0 |
| Dysphagia, n (%) | 41 (20.2%) | 124 (9.5%) | 20.78 | 0 |
| Indwelling nasogastric tube, n (%) | 73 (36.0%) | 33 (2.5%) | 299.702 | 0 |
| Initial NIHSS, median (IQR) | 8 (5-16) | 2 (1-4) | -16.374 | 0 |
| Thrombolytic therapy, n (%) | 23 (11.3%) | 138 (10.6%) | 0.262 | 0.877 |
| Length of stay, n (%) | 13 (10-18) | 10 (8-12) | -9.79 | 0 |
| Stroke position | - | - | - | - |
| The left hemisphere of the brain, n (%) | 96 (47.3) | 527 (40.5%) | 3.362 | 0.067 |
| The right hemisphere of the brain, n (%) | 88 (43.3%) | 537 (41.2%) | 0.321 | 0.571 |
| Epencephalon, n (%) | 24 (11.8%) | 73 (5.6%) | 11.253 | 0.001 |
| Diencephalon, n (%) | 8 (3.9%) | 70 (5.4%) | 0.736 | 0.391 |
| Brainstem, n (%) | 35 (17.2%) | 257 (19.7%) | 0.7 | 0.403 |
| Capsula interna, n (%) | 0 | 3 (0.2%) | 0 | 1 |
| Base node area, n (%) | 63 (31.0%) | 429 (32.9%) | 0.293 | 0.589 |
| Etiological classification | - | - | 14.265 | 0.006 |
| Large artery atherosclerosis, n (%) | 90 (44.3%) | 490 (37.6%) | - | - |
| Cardioembolism, n (%) | 41 (20.2%) | 427 (32.8%) | - | - |
| Lacunar, n (%) | 53 (26.1%) | 266 (20.4%) | - | - |
| Other known causes, n (%) | 4 (2.0%) | 35 (2.7%) | - | - |
| Undetermined, n (%) | 15 (7.4%) | 84 (6.5%) | - | - |
| Systolic pressure, median (IQR) | 146 (136-160) | 144 (130-160) | -1.111 | 0.266 |
| Diastolic pressure, median (IQR) | 84 (75-92) | 83 (77-94) | -0.808 | 0.419 |
| Creatinine, median (IQR) | 71.9 (57.6-92.4) | 73.45 (59.58-87) | -0.051 | 0.959 |
| Homocysteine, median (IQR) | 12.5 (9.7-15.64) | 11.79 (9.7-14.7) | -1.529 | 0.126 |
| Fasting blood glucose, median (IQR) | 5.94 (4.99-7.8) | 5.37 (4.8-6.5) | -3.684 | 0 |
| WBC (×109/L), median (IQR) | 9.4 (7.27-11.86) | 7.15 (5.90-8.45) | -11.176 | 0 |
| Neutrophil (×109/L), median (IQR) | 7.15 (5.16-9.2) | 4.39 (3.47-5.61) | -13.632 | 0 |
| Lymphocyte (×109/L), median (IQR) | 1.31 (0.95-1.7) | 1.93 (1.52-2.45) | -12.088 | 0 |
| NLR, median (IQR) | 5.46 (3.64-8.52) | 2.24 (1.65-3.11) | -16.172 | 0 |
| A2DS2 score, median (IQR) | 5 (3-6) | 1 (1-2) | -16.909 | 0 |
| - | OR value | 95% CI | P-value |
|---|---|---|---|
| Age | 1.034 | 1.015-1.053 | <0.001 |
| NIHSS score | 1.182 | 1.088-1.286 | <0.001 |
| NLR | 1.563 | 1.431-1.706 | <0.001 |
| A2DS2 | 1.456 | 1.207-1.757 | <0.001 |
| - | Low NLR (NLR≤ 2.43) | High NLR (NLR>2.43) | Z/χ2 | P-value |
|---|---|---|---|---|
| Number of patients | 752 | 753 | - | - |
| SAP, n (%) | 21 (2.8%) | 182 (24.2%) | 147.351 | 0 |
| Age (years), median (IQR) | 63 (54-72) | 66 (56-75) | -4.386 | 0 |
| Sex, male, n (%) | 484 (64.5%) | 507 (67.3%) | 1.475 | 0.225 |
| Stroke history, n (%) | 291 (38.7%) | 303 (40.2%) | 0.375 | 0.541 |
| Alcohol drinking, n (%) | 186 (24.7%) | 192 (25.5%) | 0.117 | 0.733 |
| Hypertension, n (%) | 456 (60.6%) | 517 (68.7%) | 10.591 | 0.001 |
| Diabetes, n (%) | 193 (25.7%) | 225 (29.9%) | 3.393 | 0.064 |
| Hyperlipidemia, n (%) | 64 (8.5%) | 55 (7.3%) | 0.739 | 0.390 |
| Coronary disease, n (%) | 66 (8.8%) | 77 (10.2%) | 0.852 | 0.356 |
| Atrial fibrillation, n (%) | 31 (4.1%) | 70 (9.3%) | 0.441 | 0.507 |
| Cerebral infarction, n (%) | 143 (19.0%) | 154 (20.5%) | 0.838 | 0.360 |
| Hematencephalon, n (%) | 16 (2.1%) | 33 (4.4%) | 6.073 | 0.014 |
| COPD, n (%) | 11 (1.5%) | 19 (2.5%) | 2.166 | 0.141 |
| Disturbance of consciousness, n (%) | 30 (4.0%) | 65 (8.6%) | 13.714 | 0 |
| Dysphagia, n (%) | 67 (8.9%) | 98 (13.0%) | 6.450 | 0.011 |
| Indwelling nasogastric tube, n (%) | 23 (3.1%) | 83 (11.0%) | 36.450 | 0 |
| Initial NIHSS, median (IQR) | 2 (1-4) | 3 (2-5) | -9.537 | 0 |
| Thrombolytic therapy, n (%) | 76 (10.1%) | 85 (11.3%) | 0.537 | 0.464 |
| Length of stay, n (%) | 9 (8-11) | 10 (8-13) | -5.868 | 0 |
| Systolic pressure, median (IQR) | 143 (130-158) | 147 (133-162) | -3.615 | 0 |
| Diastolic pressure, median (IQR) | 82 (7-93) | 85 (77-95) | -1.794 | 0.07 |
| Creatinine, median (IQR) | 70.1 (58.6-83.85) | 76.3 (61.1-91.45) | -4.632 | 0 |
| Homocysteine, median (IQR) | 11.5 (9.6-13.98) | 12.2 (9.7-15.85) | -3.971 | 0 |
| Fasting blood glucose, median (IQR) | 5.35 (4.8-6.49) | 5.51 (4.84-6.85) | -1.572 | 0.116 |
| WBC (×109/L), median (IQR) | 6.7 (5.58-7.99) | 8.07 (6.55-9.81) | -3.115 | 0 |
| Neutrophil (×109/L), median (IQR) | 3.74 (3.05-4.57) | 5.76 (4.64-7.56) | -3.282 | 0 |
| Lymphocyte (×109/L), median (IQR) | 2.2 (1.82-2.74) | 1.49 (1.16-1.88) | -1.663 | 0 |
| A2DS2 score, median (IQR) | 1 (1-2) | 2 (1-4) | -9.537 | 0 |
| - | AUC (95%CI) | P-value | Best Cut-off value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| A2DS2 | 0.855 (0.822-0.888) | 0 | 3.5 | 0.733 | 0.861 |
| NLR | 0.849 (0.816-0.882) | 0 | 3.44 | 0.797 | 0.806 |
| A2DS2 and NLR | 0.924 (0.904-0.944) | 0 | 0.1 | 0.871 | 0.828 |
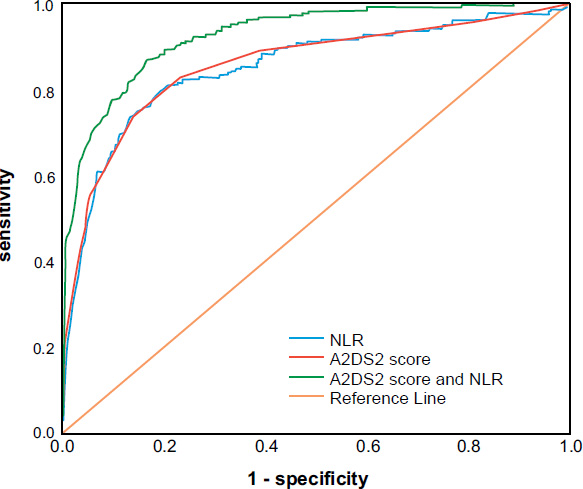
3.3. Prediction Effect of the A2DS2 Score and NLR on SAP
The results showed that the A2DS2 score predicted the area under the curve (AUC) of SAP as 0.855 (95% confidence interval (CI): 0.822–0.888, P=0.000), with 73.3% sensitivity, 86.1% specificity, and an optimal cut-off value of 3.5. The NLR predicted an AUC of 0.849 (95% CI: 0.816–0.882, P=0.000), a sensitivity of 79.7%, a specificity of 80.6%, and an optimal cut-off value of 3.44. The A2DS2 score combined with NLR predicted an AUC of 0.924 (95% CI: 0.904–0.944, P=0.000), a sensitivity of 87.7%, and a specificity of 82.8% for SAP (Fig. 1 and Table 5).
4. DISCUSSION
AIS refers to softening and necrosis of local brain tissue due to blood circulation disorders, ischemia, and hypoxia. Acute onset occurs with a high rate of disability and death. SAP is one of the most common complications in the acute stage of stroke and is an important factor leading to poor prognosis and increased economic burden in patients with stroke. In our study, SAP incidence was 13.5%, which was consistent with previous studies [14, 18, 19]. We also observed that age, hypertension and COPD history, consciousness disturbance, dysphagia, indwelling nasogastric tube, NHISS score, and fasting blood glucose level were risk factors for SAP. WBC count, neutrophil count, and NLR were significantly higher in the SAP group than in the non-SAP group, whereas the lymphocyte count was lower in the SAP group than in the non-SAP group. Conversely, the A2DS2 score was significantly higher in the SAP group than in the non-SAP group, and patients with SAP had a significantly longer hospital stay. Multivariate logistic regression analysis revealed that age, NIHSS score, NLR, and A2DS2 score were independent risk factors for SAP. We also divided 1,505 patients into a low NLR group (≤2.43) and a high NLR group (>2.43). A high NLR was associated with a higher SAP incidence and NIHSS score.
Aspiration is implicated in the development of aspiration pneumonia. As patients grow older, their resistance and protective reflexes weaken, and they become prone to coughing, which can lead to pneumonia [20, 21]. Pneumonia incidence in patients with dysphagia after stroke is three times higher than in patients without dysphagia. The risk of pneumonia in patients diagnosed with dysphagia is 11 times higher [22]. Therefore, early screening of swallowing function can reduce the incidence of stroke-associated pneumonia and improve stroke outcomes [23]. An indwelling nasogastric tube is used to reduce aspiration; however, it does not reduce SAP incidence. The reason may be that the gastric tube placement may lead to abnormal contraction and closing of the lower esophageal sphincter, gastric content regurgitation, and aspiration. Some researchers believe that severe hypertension on admission (≥200/120 mmHg) is an independent risk factor for SAP in older adults with AIS [24]. The results of this study showed that there was no significant difference in blood pressure on admission between the SAP and non-SAP groups, possibly because we did not classify blood pressure on admission. However, the proportion of patients with a history of hypertension was higher in the SAP group than in the non-SAP group. In addition, the SAP group had a high proportion of patients with COPD, which may be due to the patients’ poor pulmonary function, chronic pathological changes in the bronchi and lung tissues, and acute exacerbation when immunity is low. Fasting blood glucose levels in the SAP group were higher; this may be because a high blood glucose environment is conducive to bacterial growth and reproduction, increasing the risk of infection.
The post-stroke immunosuppressive response is closely related to SAP occurrence. Systemic immunosuppression caused by an acute stroke can protect the brain from further inflammatory stimulation; however, it also increases the risk of post-stroke infection [25]. The NLR is an economical and simple inflammatory marker with a higher predictive value for SAP than conventional single leukocytes, neutrophils, lymphocytes, and hypersensitive C-reactive proteins [14, 26]. We discovered that NLR is associated with SAP; a high NLR was directly correlated with a high SAP incidence. The association is explained as follows: the first explanation is post-stroke-related immunosuppression. AIS leads to local brain damage, triggering a local inflammatory response followed by a systemic inflammatory response via sympathetic pathways and the hypothalamic-pituitary-adrenal axis. Neutrophils are dedifferentiated and stimulated by growth factors, whereas lymphocytes undergo apoptosis [25, 27]. Second, the NLR is related to stroke severity and size. The more severe the stroke and the larger the area involved, the more likelihood of an infection occurring [14]. In this study, we observed that patients with high NLR were more likely to merge SAP with a higher NIHSS score. Third, the NLR is believed to be associated with various diseases, such as hypertension, renal insufficiency, cardiovascular disease, cancer, etc. [28-31]. Chronic diseases tend to increase the risk of infection. We also discovered that patients with a high NLR were more likely to have a history of hypertension and intracerebral hemorrhage, and serum creatinine and homocysteine levels were also associated with a higher NLR.
The A2DS2 score is an SAP risk prediction scale that can be completed upon admission, with a total score of 10 points. The higher the score, the higher the risk of pneumonia. Multiple studies have confirmed that the A2DS2 score has good predictive value for SAP [7-9, 32, 33]. The results of this study showed that taking 3.5 as the optimal cut-off value, the sensitivity of A2DS2 to predict SAP was 73.3%, the specificity was 86.1%, and the AUC was 0.855. A meta-analysis study showed that compared with AIS-AP and ISAN scores, A2DS2 scores showed a more stable cut-off value, suggesting that AIS-AP and ISAN scores could not accurately identify high-risk SAP [34]. Although A2DS2 has a high predictive value for SAP, it lacks the support of quantified inflammatory indicators. In this study, the A2DS2 score and NLR were combined to evaluate the predictive value of SAP. The results showed that the AUC (0.924) of the combined prediction of SAP was significantly higher than that of the single A2DS2 score (AUC: 0.855) and NLR (AUC: 0.849), and the sensitivity of the prediction was also remarkably improved. This result indicates that the predictive value of the combination of the two indices is better than that of a single indicator.
This study had some limitations. First, the study participants were retrospectively recruited from a single center, and there was a certain selection bias. Second, the relationship between NLR and SAP may differ at different periods without dynamic monitoring of NLR changes. Third, we selected patients with AIS who were admitted to the hospital within 48 hours of onset as the study participants, but some patients may still be missing. Finally, SAP severity was not evaluated, and the relationship between the NLR and SAP was unclear.
CONCLUSION
Our study showed that the A2DS2 score combined with NLR had a higher predictive value and sensitivity for SAP, reaching 87.7% compared to the A2DS2 score or NLR alone. As a predictive scale for early SAP risk, A2DS2 can be quickly completed upon admission, and the NLR can be quickly and easily obtained by routine blood detection. The combination of the two can predict SAP more accurately and help to identify high-risk patients with SAP early and commence intervention measures, thus contributing to the functional recovery of patients with stroke and improving their prognosis.
AUTHORS’ CONTRIBUTIONS
Chunhua Liang contributed to data Curation, formal analysis, and writing of the Original Draft. Xiaoyong Xiao contributed to conceptualization and methodology; Xiaohua Xiao contributed to writing, review and editing; Xueqin Yan, Huoyou Hu, Jing Tian, and Cuimei Wei contributed to Software Validation.
LIST OF ABBREVIATIONS
| SAP | = Stroke-associated Pneumonia |
| NLR | = Neutrophil-to-lymphocyte Ratio |
| NIHSS | = National Institute of Health Stroke Scale |
| WBC | = White Blood Cell |
| ROC | = Receiver Operating Characteristic |
| AUC | = Area under the Curve |
| AIS | = Acute Ischemic Stroke |
| COPD | = Chronic Obstructive Pulmonary Disease |
| A2DS2 | = Age, Atrial fibrillation, Dysphagia, Sex, Stroke Severity |
| AIS APS | = Acute Ischaemic Stroke-associated Pneumonia Score |
| ISAN score | = Prestroke Independence, Sex, Age, National Institutes of Health Stroke Scale |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Clinical Research Ethics Committee of the Second People's Hospital of Shenzhen (NO:2023-126-02PJ) approved this study.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This study was supported by Shenzhen Municipal Science and Technology Innovation Commission for Sustainable Development (KCXFZ20201221173213036).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


