All published articles of this journal are available on ScienceDirect.
Complications of Acute Kidney Injury in COVID-19 Patients: A Systematic Review
Abstract
Introduction:
Acute Kidney Injury has been associated with a higher mortality rate among hospitalized patients with Coronavirus disease.The present review aimed to evaluate the association of COVID-19 with acute kidney injury. The study also aimed to assess the symptoms, complications, and treatment performed for the successful management of acute kidney injury patients with COVID-19.
Methodology:
The literature review search was conducted by using PubMed, Medline, and another database of medical journals for identifying, reviewing, and evaluating the published articles with cases of COVID-19 and acute kidney injury complications.
Results:
The systematic review of 25 studies found that patients with COVID-19 had a high prevalence of acute kidney injury due to risk factors, such as hypertension, diabetes, cardiovascular disease, and the overuse of diuretics. The average age of acute kidney injury occurrence in patients was from 54-70 years of age. From 25 studies, a total of 27922 patients, 29.9%, were detected with acute kidney injury with the pathological cause of acute tubular necrosis and focal segmental glomerulosclerosis.
Conclusion:
The current systematic review indicates a high prevalence of acute kidney injury among COVID-19 patients with hospitalisation. Patients with COVID-19 pose a risk for the development of acute kidney injury due to chronic infection, high use of corticosteroids, and systemic hemodynamic instability, which results in acute tubular injury in hospitalised COVID-19 patients.
A majority of the patients were seen with undiagnosed acute kidney injury. Early detection in such comorbid cases can resolve renal complications and improve the therapeutic outcome in COVID-19 patients.
1. INTRODUCTION
Acute kidney injury has been reported preliminary in hospitalised patients with COVID-19. Small studies from China, Europe, and the United States have reported a high rate of incidence for AKI, ranging from 1% to 42%. The retrospective observational study has shown that out of 3993 hospitalized patients, AKI was seen in 46% of the patients, and 19% required dialysis. The severity of AKI increases in patients with COVID-19 and results in increased mortality among patients [1].
The severity of the AKI depended on the etiology, age, comorbidities, and clinical condition of the hospitalised patient. A larger cohort study has stated that the prevalence of kidney disease is associated with in-hospital death, with a median time to death of 6 days in COVID-19 patients [2]. Data collected from a prospective study indicated that most of the patients with COVID-19 were found with comorbid conditions, such as cardiovascular disease, hypertension, diabetes, and chronic pulmonary disease and were more prone to AKI and high mortality conditions [3, 4].
The diagnosis of AKI among hospitalised patients can be assessed by using the Kidney Disease Improving Global Outcomes (KDIGO) criteria. A prospective cohort study in 101 hospitalised patients revealed a 50% incidence of AKI with 65.4% of mortality with a significant correlation with causative factors, such as obesity [5-7]. Another observational study has revealed that male sex and older age were significant factors for the development of AKI [8]. In addition, another study has revealed that patients with COVID-19 can develop AKI during their hospitalization, with the peak as stage 1, stage 2, or stage 3 requiring renal replacement therapy. The fatality and severity have also been seen to be dependent on AKI. The study stated the loss of 694 patients (35%) along with poor prognosis in comorbidity situations. AKI is one of the markers for severe disease complications in hospitalised patients, especially patients admitted to the ICU who have seen a high prevalence of AKI [9, 10].
Several studies have shown a significant association between COVID-19 infection and the progression of AKI as a comorbid condition. Patients presented in the majority of the studies are diagnosed with proteinuria and hematuria, which is one of the clinical findings related to AKI [2, 8, 10].
The review aims to evaluate and compare the symptoms, complications, clinical management, criteria of AKI, and treatments given for hospitalised patients with COVID-19 and AKI. The study also compares the prognosis factors associated with AKI as a comorbid condition in hospitalised patients with COVID-19.
2. MATERIALS AND METHODS
The review was conducted by using standard methods for identifying, collecting, analysing, and interpreting data from several articles and published papers.
2.1. Literature Review
The literature review search was conducted on PubMed/Medline for identifying published data from the year 2020 to 2022.
The following keywords were used for the search strategy: COVID-19, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, and acute kidney injury. Studies with written English were selected.
2.2. Study Selection
The data selected from the database were merged and identified for duplications. The duplicates were removed by the reviewers. The title/abstract and full texts were independently screened from the database to exclude unrelated articles from the study objectives.
2.3. Study Screening
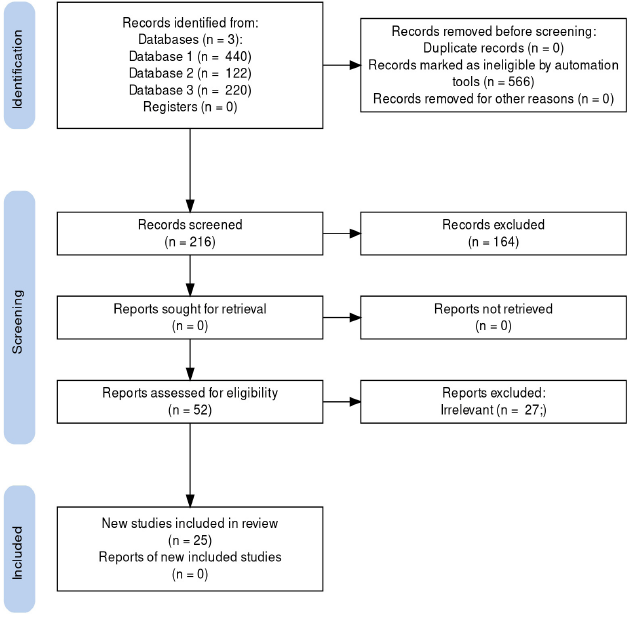
A total of 782 studies were identified in the initial database literature. After an in-depth review, 566 studies were excluded due to title/abstract and full-text review. A total of 216 full-text articles were screened (Fig. 1). Of these, 52 articles were screened for full-text review. After the full-text review, a total of 25 articles met the inclusion criteria.
Fig. (1) denotes the overall selection criteria and methodology for the review article.
3. RESULTS
A total of 25 studies were included in the review article, which characterises the prevalence of AKI in hospitalised patients diagnosed with COVID-19. Table 1 indicates the characteristics, staging, and mean age of the patients for the included studies.
In the total of studies, patients diagnosed with COVID-19 and AKI were in the age range of 54-70 in a majority of the studies and showed a high prevalence of Stage 1 and Stage 3 types of AKI in hospitalised patients.
| - | Study | Year | Country | Design | Sample Size | Mean Age | No: of Patients with COVID-19 & AKI | Diagnosis Criteria OF AKI | Department |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chan, et al. [1] | 2020 | USA | Observational retrospective study | 3993 | 64 | 1835 | Stage 1=39% Stage 2= 19% Stage 3= 42% |
Hospitalized |
| 2 | Chenna, et al. [2] | 2020 | USA | Retrospective cross sectional study | 3235 | 66.5 | 815 | - | Hospitalized |
| 3 | Yang, et al. [3] | 2020 | China | Single centered,Retrospective- observational study | 52 | 59.7 | - | 2012 KDIGO criteria | ICU |
| 4 | Yu, et al. [4] | 2020 | China | Multicenter prospective- observational study | 226 | 64 | 57 | Stage 1 (n) = 23 Stage 2 (n) =12 Stage 3 (n) = 22 |
ICU |
| 5 | Wang, et al. [5] | 2020 | China | Retrospective cross sectional study | 116 | 54 | - | 2012 KDIGO Criteria | Hospitalized |
| 6 | Arentz, et al. [6] | 2020 | USA | Retrospective cross sectional study | 21 | 70 | - | 2012 KDIGO Criteria | ICU |
| 7 | Zamoner, et al [7]. | 2021 | Brazil | Prospective cohort study | 101 | 57.07 | 66.6% | Stage 1= 27% Stage2=36.6% Stage 3=49.1% |
Hospitalized |
| 8 | Fisher, et al. [8] | 2020 | USA | Retrospective observational study | 3345 | 71 | 1903(56.9%) | Stage 1=942 Stage 2=387 Stage3=574 |
Hospitalized |
| 9 | Hirsch, et al. [9] | 2020 | New York | Retrospective observational cohort study | 5449 | 64 | 1993 | Stage1=46.5% Stage2=22.4% Stage3=31.1% |
Hospitalized |
| 10 | Thakkar, et al [10] | 2020 | USA | Retrospective observational study | 300 | - | 224 | Stage1=22% Stage 2=16% Stage3=63% |
ICU |
| 11 | Ferlicot, et al [11] | 2020 | Paris | Retrospective study | 47 | 63 | 30 | Stage 1=6.5% Stage2=4.3% Stage3=87.2% |
Hospitalized |
| 12 | Gabarre, et al [12] | 2020 | China | Case series | 116 | 63 | 42 | - | ICU |
| 13 | Dai, et al [13] | 2020 | China | Retrospective study | 492 | 69 | 36 | Stage 1=72.2% Stage2=5.6% Stage3=22.2% |
Hospitalized |
| 14 | Hong, et al [14] | 2020 | S. Korea | Retrospective Cross sectional study | 98 | 55.4 | - | 2012 KDIGO Criteria | Hospitalized |
| 15 | Hamilton et al [15] | 2020 | UK | Retrospective study | 1032 | 71 | 210 | Stage1:58% Stage2:18% Stage3:24% |
Hospitalized |
| 16 | Zheng, et al. [16] | 2020 | China | Retrospective study | 34 | 66 | - | - | ICU |
| 17 | Goldman, et al [17] | 2020 | Taiwan, US, Germany, Singapore, Italy, Spain S.Korea, Hong Kong |
Randomized, Open labeled Phase 3 Study | 397 | 61-62 | - | - | Hospitalized |
| 18 | Richardson, et al. [18] | 2020 | USA | Case Series | 5700 | 63 | - | - | Hospitalized |
| 19 | Regina, et al [19] | 2020 | Switzerland | Retrospective observational study | 200 | 62 | 2012 KDIGO Criteria |
2012 KDIGO Criteria |
Hospitalized |
| 20 | Shi, et al [20] | 2020 | China | Cohort Study | 416 | 64 | - | 2012 KDIGO Criteria |
Hospitalized |
| 21 | Liu, et al. [21] | 2020 | China | Retrospective | 920 | 72 | - | - | Hospitalized |
| 22 | Rubin, et al. [22] | 2020 | France | Single Center Cohort | 71 | - | 57 | Stage 1:35% Stage2: 35% Stage 3: 30% |
ICU |
| 23 | Su., et al [23] | 2020 | China | Observational Study(Autopsy) | 26 | 69 | 9 | - | Death |
| 24 | Xiao. et al [24]. | 2020 | China | Single-center, Retrospective Observational study | 287 | - | 55 | Stage1:14.3% Stage2: 4.9% Stage3: 4.9% |
Hospitalized |
| 25 | Jewell, et al [25]. | 2020 | UK | Retrospective Cohort study | 1248 | 69 | 487 | Stage1: 51% Stage2: 13% Stage3: 36% |
Hospitalized |
The demographic details based on admission are included in Table 2 which portrays the prevalence of AKI on admission, history of diabetes, hypertension, and cardiovascular disease. AKI was prevalent during admission in a total of 7799 patients diagnosed with COVID-19 by interpreting data from 12 studies. In addition, 5 studies have stated that 561 patients developed AKI after hospitalisation. Data from 14 studies revealed that 3218 patients had a history of diabetes, and 11 studies had shown that 3621 patients were detected with a history of hypertension. About 208 patients from 9 studies have shown a significant history of chronic kidney disease.
| Variables | Number of Studies | Number of Patients with COVID-19 and AKI |
|---|---|---|
| Demographic Details of the Patients | ||
| AKI during admission | 12 | 7799 |
| AKI after admission | 5 | 561 |
| History of Diabetes | 14 | 3218 |
| History of Hypertension | 11 | 3621 |
| Chronic kidney disease | 9 | 208 |
| Signs and Symptoms of Patients | ||
| Fever | 7 | 684 |
| Cough | 4 | 221 |
| Shortness of Breath | 4 | 191 |
| Myalgia | 3 | 49 |
| Diarrhoea | 3 | 15 |
| Fatigue | 2 | 57 |
| Headache | 2 | 11 |
| Sore Throat | 1 | 12 |
| Comorbid Conditions Reported in Patients | ||
| COPD | 9 | 297 |
| Cancer | 6 | 185 |
| HIV | 4 | 15 |
| Hepatitis B | 1 | 4 |
| Pregnancy | 1 | 7 |
| Diabetes | 14 | 3218 |
| Hypertension | 11 | 3621 |
| Prognosis of the Patient | ||
| ICU admission | 8 | 2816 |
| Ventilation requirement | 10 | 2220 |
| Septic Shock | 1 | 34 |
| Variables | Number of Studies | Number of Patients with COVID-19 and AKI |
|---|---|---|
| Fever | 7 | 684 |
| Cough | 4 | 221 |
| Shortness of Breath | 4 | 191 |
| Myalgia | 3 | 49 |
| Diarrhoea | 3 | 15 |
| Fatigue | 2 | 57 |
| Headache | 2 | 11 |
| Sore Throat | 1 | 12 |
| Variables | Number of Studies | Number of Patients with COVID-19 and AKI |
|---|---|---|
| COPD | 9 | 297 |
| Cancer | 6 | 185 |
| HIV | 4 | 15 |
| Hepatitis B | 1 | 4 |
| Pregnancy | 1 | 7 |
The study categorises the symptoms seen in patients in Table 3. Depending on various conditions diagnosed in the patient. A majority of the patients were seen fever as the common symptom (684 patients reported in 7 studies), followed by cough (221 patients reported in 4 studies) and shortness of breath (191 patients in 4 studies). Other symptoms, such as myalgia, were also seen in 49 patients, as reported in 3 studies. Less common symptoms include fatigue, headache, sore throat, and oedema.
As shown in Table 4, the prevalence of comorbid conditions was high in hospitalised patients. Hypertension and diabetes were the most prevalent diseases found in the studies. Chronic pulmonary obstructive disease (COPD) was found to be the third most reported disease in 9 studies, accounting for 297 patients, which was followed by cancer in 185 patients from 6 studies. In addition, Human Immunodeficiency Virus (HIV) was seen in 4 studies, including a total of 15 patients.
The clinical condition of the hospitalised patients is demonstrated in Table 5, which includes a significant number of patients (2816) admitted to the ICU, as reported in the 8 studies. Patients generally admitted to ICU required ventilation, which was reported in 10 studies accounting for a total of 2220 patients. The prevalence of septic shock was only reported in 1 study, which accounted for 34 patients.
From a total of 25 studies, 6 studies reported a loss of life for 735 patients, and 3 studies reported adult respiratory distress syndrome in 209 patients.
Table 5.
| Variables | Number of Studies | Number of Patients with COVID-19 and AKI |
|---|---|---|
| ICU admission | 8 | 2816 |
| Ventilation requirement | 10 | 2220 |
| Septic Shock | 1 | 34 |
4. DISCUSSION
In the present systematic review, a total of 7799 patients were found with COVID-19 and AKI before hospitalisation, and 561 patients developed AKI after hospital admission. The data can be supported by the cohort study conducted by Chan et al., which also reported the prevalence of AKI in COVID-19 patients accounting for 1835 patients (46%) [1]. This can be correlated to the high prevalence of AKI in patients diagnosed with COVID-19 infection. The association of AKI in COVID-19 has been associated with regional inflammation, endothelial injury, and renal microthrombi; however, a clear understanding of the pathogenesis remains unclear [2, 3].
The study also reported a strong prevalence of AKI in patients with comorbid conditions, such as diabetes or hypertension. However, the incidence of AKI in the study was higher than the reported incidence in China and Italy. Patients with COVID-19 infection with elevated serum creatinine developed AKI during the hospitalisation stage, which can be due to multiple factors related to treatment, the severity of the infection, and an unstable hemodynamic state [1, 2].
The pathogenesis of AKI in COVID-19 patients is considered multifactorial. Recent studies have shown the association of angiotensin-converting enzyme 2 (ACE2) as a dependent pathway for inducing immunological reactions in the kidney. Age is one of the factors associated with AKI in hospitalised patients, the retrospective study conducted by Cheng et al., has revealed a mean age of 59.7 for the prevalence of COVID-19 and AKI as a comorbid condition [2]. The study conducted by Cheng et al. has revealed that patients with older age and diagnosed with AKI during admission have higher chances of in-hospitalization death [2].
The most common symptoms presented among the hospitalised patients were fever, cough, sore throat, and dyspnoea, which were prevalent in the majority of the studies. Concomitant comorbidity in the systematic review shows that most of the patients were presented with hypertension, diabetes, or cardiovascular disease. Based on the systematic review, 11 studies reported that hospitalised patients were presented with hypertension (3621 patients), and 14 studies showed that patients had a strong history of diabetes (3218 patients). Several studies have evaluated the presence of comorbidity as a significant risk factor for the development of AKI [4, 5].
The association of hypertension and diabetes in COVID-19 patients shows a higher risk for AKI as a major morbid condition, as a significant number of patients presented with a history of hypertension (3621 overall) and diabetes (3218 overall). Literature has suggested that a history of hypertension or diabetes is the risk factor associated with the development of AKI in COVID-19 patients [7-10]. This can be due to the concomitant use of multi-drug therapy and poor functioning of the organs due to comorbid conditions.
The pathological assessment conducted by Ferlicot et al. revealed two significant patterns of kidney damage among the patients: tubulointerstitial injury and glomerular injury, respectively [11]. A few patients also showed focal segmental glomerulosclerosis (FSGS). The presence of FSGS is majorly prevalent in critically ill patients with comorbid conditions [12].
Independent risk factors in COVID-19 patients were secondary bacterial infection, overuse of diuretics, glucocorticoids, and hypertension, which led to the development of AKI during their hospital stay. These factors were more prevalent in the patients stated by Dia et al. in a single-center hospital-based study among 492 patients [13].
Patients with COVID-19 and AKI had high mortality when compared with patients without AKI. This is due to the comorbid conditions and the significant effect of AKI on the patient’s disease progression. Several studies have shown that patients with AKI and COVID-19 infection are more likely to be admitted to ICU and require ventilation due to poor prognosis [14, 15]. In addition, the survival rate of the patients can be increased if they survive through the ICU stage of hospitalisation. The study conducted by Hamilton et al. has shown that among 38 patients, 21 died during their ICU stay, and 2 lost their lives post-ICU admission [15-21]. This data suggest that patient management during the ICU stay is a critical step in tackling the comorbid condition. The mortality and death were seen to be significantly higher in patients presented with COVID-19 and AKI altogether, whereas patients who were presented with COVID-19 infection showed a better survival rate [22-25].
In recent studies, kidney tissue sample analysis had posed a potential on the pathophysiology responsible for COVID-19-related AKI. The mechanism includes tubular epithelial and podocyte damage due to ACE2, direct infection of glomerular endothelia, hypovolemia related to hypovolemia, which can lead to pre-renal AKI, activation of cytokine storm, hypercoagulability, and multiple organ damage. The other form of etiology can be due to the use of nephrotoxic drugs or contrast media [26-29].
The mortality of AKI is one of the independent factors which is seen across all stages of severity, including stage 1. The data from the cohort study conducted in the United States among 13 hospitals have stated that patients with AKI required ICU and renal replacement therapy, and patients with AKI had a 3-fold higher risk of death when compared to patients without AKI. However, 84% of the patients from the cohort study were discharged with adequate therapy and treatment plans [25-35].
Patients presented with COVID-19, with or without the prevalence of AKI, received the standard care of treatment and isolation. Moreover, patients with AKI received diuretic treatment for heart failure or oedema. The empirical treatment of antibiotic and antiviral treatment was seen in all the patients admitted to the ICU. Patients with AKI received more systemic corticosteroids when compared with patients without AKI [13, 14, 16]. The use of corticosteroids and diuretics has been shown to have improved outcomes in patients with less severe AKI [13, 36-48].
The use of remdesivir was also seen for the course of 5 to 10 days among ICU-admitted patients with COVID-19 and AKI. The 5-day course treatment was shown to have a beneficial effect on patients with mild to moderate severity of AKI. Patients on mechanical ventilation saw improvement with a 10-day course of remdesivir. However, an elevation of the liver enzyme was seen in both groups [15]. The use of remdesivir should be based on the clinical condition and history of the patient [49-55].
The systematic review indicates that COVID-19-related AKI may have long-term effects on kidney function and overall health. AKI survivors from COVID-19 may be more likely to develop chronic kidney disease (CKD) or endure a progressive decline in kidney function, according to certain research. COVID-19 is one of the essential factors that can result in AKI among patients who are 1) diagnosed with hypertension or diabetes, 2) older in age, and 3) diagnosed with other complications or underlying infections. Older age men were more prevalent with the clinical presentation of AKI, which is one of the independent factors for the progression of the disease. Hence, early screening in such instances can be beneficial to control the progression of AKI stages among individuals diagnosed with COVID-19.
Though AKI in COVID-19 poses a high mortality situation, certain treatment approaches should be assessed before initiating the treatment in the patient. The lack of effective treatment for patients with COVID-19 and AKI has required the revaluation of medications, including Remdesivir. The length of hospital stay is longer in such patients; hence, there is an utmost need for an adequate treatment plan.
The limitations of the systematic review are the consistent data findings for hospitalisation stay and clearly indicated treatment. In addition, the use of corticosteroids is yet to be explored in patients with AKI and COVID-19. The published articles lacked comprehensive patient characteristics information, making it difficult to look into the potential impact of pre-existing conditions. Other restrictions included the diversity of investigations and the features of various patients.
CONCLUSION
This systematic review indicates that AKI is one of the most frequent complications during the course of COVID-19 hospitalisation which is strongly associated with high mortality, prolonged hospital stay, poor prognosis, and increased risk of death. The mortality rates are higher in patients with COVID-19 infection and AKI when compared with patients with non-AKI complications.
In clinical practice, this review is crucial. It offers a thorough overview, identifies prevalence and risk factors, investigates underlying mechanisms, highlights the necessity for multidisciplinary care, and gives information for clinical guidelines creation. This review improves patient outcomes by facilitating evidence-based decision-making and expanding our understanding of AKI in COVID-19 by synthesising the current research.
The literature has seen undiagnosed conditions of AKI in COVID-19, which pose a higher risk of complications. Nonetheless, early detection of comorbidities and renal complications can improve the patient outcome in COVID-19 patients.
LIST OF ABBREVIATIONS
| AKI | = Acute kidney injury |
| COPD | = Chronic obstructive pulmonary disease |
| HIV | = Human Immunodeficiency Virus |
| KDIGO | = Kidney Disease Improving Global Outcomes |
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.
Supplementary material is available on the publisher’s website along with the published article.


