All published articles of this journal are available on ScienceDirect.
Analgesic Equivalent Days: A Novel Approach to Assess Chronic Analgesic Use in the Population
Abstract
Introduction:
There is no simple method to compare the use of multiple analgesic products in mild to moderate pain. The aim of this study is to validate a new tool to assess analgesic use.
Methods:
A measure of Analgesic Equivalent Days (AEDs) was developed using Defined Daily Doses (DDD) and the total mg of each analgesic over a 12 month period. Comparisons were made using analgesic class and all analgesics combined.
Results:
In a group of newly initiated patients with Osteoarthritis, AEDs values indicated that patients received around 70% of AEDs from paracetamol, 20% from NSAIDs and 10% from opioids. AEDs were similar between the two paracetamol formulations. However, one group took 8 more AEDs of NSAIDs, while the other group took 7 more AEDs of opioids.
Conclusion:
Even though the total AED scores, there was no significant difference in total analgesic use between the two formulations, differences were found among the analgesic classes. The AED methodology was sufficiently sensitive to demonstrate that one group of patients climbed higher up the analgesic ladder than the other group. AEDs are easy to calculate and seem to produce valid outcomes from both a statistical and a clinically meaningful perspective.
1. INTRODUCTION
There are many issues when assessing the use of analgesics in the general population. In particular, it is difficult to adequately assess analgesic use over time because patients take multiple analgesic products in various strengths and pack sizes. These analgesics can be taken chronically or acutely and for different indications, as well as for differing durations of time.
A review of 273 studies failed to find clear differences in efficacy for pain relief in treating Osteoarthritis (OA) associated with different NSAIDs [1]. There are oral Morphine Equivalent Daily Doses [2, 3], however, there is no method to combine and compare multiple analgesic products in mild to moderate pain.
The Australian prescription claims database has been used to assess medication use in defined patient populations [4-8]. Each product has a unique PBS identification code and some of these codes are restricted to specific indications. While the PBS prescription claims database can be used to determine the number of patients taking prescription analgesic products, these data do not include products costing less than the patient co-payment or Over The Counter (OTC) analgesics [4]. The Australian prescription claims data do not indicate the dose of analgesic taken. Information on the number of prescriptions for each product and the date they were dispensed is available. Using prescription counts has limitations because a pack of 20 paracetamol 500mg and codeine 30mg tablets (3-4 days of treatment) is weighted the same as one pack of 50 capsules of celecoxib 200mg (50 days of treatment). Therefore, combining raw prescription counts across products can be misleading. Similarly, determining the proportion of patients receiving a drug does not explain how much drug a patient has taken. In addition, there is no simple method to interpret analgesic use with multiple classes of analgesic drugs over time.
The Analgesic Equivalent Day (AED) method was developed to overcome differences in product strength and pack size issues with a common metric so that multiple classes of analgesics could be combined to derive a measure of total analgesic use [4].
AEDs are derived by multiplying the pack size (number of tablets) by the product strength to determine the number mg in a product pack. The number of mg per pack is multiplied by the number prescriptions to derive the total mgs for each drug for the time period. The total number of mgs for each drug is divided by its Defined Daily Dose (DDD) and this gives a number of AEDs by molecule. AEDs of individual molecules are summed to derive the total AEDs for each analgesic class and the AEDs values for molecules can be summed to derive a total number of AEDs for the time period. That is, the AED value for each molecule has the same denominator (analgesic days), so AEDs can be summed within and across analgesic classes. In contrast, values from patient numbers per drug and prescription counts per drug should not be combined across analgesic classes.
The Defined Daily Dose (DDD) is a statistical measure of drug consumption, defined by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology [9]. It is defined in combination with the ATC Code drug classification system for grouping related drugs. The DDD enables comparison of drug usage among different drugs in the same group or among different health care environments, or to look at trends in drug utilisation over time.
That is, the number of analgesic equivalent days (AED) using WHO Defined Daily Dose (DDD) [9] are calculated for each patient as follows: AEDs = (strength (mg) x quantity x number of scripts)/DDD = (total number of mg) /DDD were calculated for each analgesic molecule. For example, paracetamol has a DDD of 3,000mg, so a single prescription for paracetamol 500mg x 300 tablets would result in 500mg x 300 / 3000 mg/day = 50 analgesic equivalent days. AEDs can be summed by a molecule and then summed within each analgesic class, and a total AED score can be derived by summing AEDs for all appropriate analgesic molecules dispensed over the time period.
The aim of this study was to explore the sensitivity and validity of the AED methodology by comparing AEDs values with: the proportion of patients receiving analgesics; the proportion of prescriptions; and for each analgesic class in a population of older Australian patients with Osteoarthriti (OA).
Two paracetamol formulations were listed on the PBS in Australia (with unique identification codes for OA): paracetamol extended-release 665 mg x 192 tablets and paracetamol immediate release 500mg x 300 tablets. The extended-release (ERP) formulation provides a longer duration of pain relief than the immediate release IRP formulation, so this original research hypothesis [4] was that there would be greater use of other analgesics associated with the IRP formulation than with the ERP formulation.
2. METHODS
The responsiveness of the AED methodology was assessed on two non-randomised groups of older patients initiated on different formulations of paracetamol (sustained release and immediate release) for OA. Since both groups were initiated on the same analgesic, large differences in analgesic use between the two formulations were not expected.
2.1. Data Source
The Pharmaceutical Benefits Scheme (PBS) prescription claims data from a 10% random sample of the Australian population was provided by the Department of Human Services (DHS) (described in more detail in a previous study [4]). Patient identities remained anonymous and approval was obtained from the Medicare Australia External Request Evaluation Committee (EREC). The committee considers requests against privacy and secrecy considerations and resource use.
All long term concession cardholders, who were received at least one prescription for OA paracetamol in the time window January 2009 to December 2010, were selected, and they were aged between 50 to 85 years. The index prescribing event was the first OA paracetamol script dispensed after January 1, 2009, and before December 31, 2010. New initiations of OA paracetamol were selected (defined as not having a prescription for OA paracetamol in the previous 12 months of the index OA paracetamol prescription). Patients who had received OA paracetamol within 12 months prior to the index prescription or patients receiving drugs for cancer or rheumatoid arthritis were excluded from this analysis.
Based on the WHO analgesic ladder categories [10], analgesic drugs were divided into six analgesic classes: Paracetamol, Cyclooxygenase-2 Inhibitors (COX-2I), NSAIDs, mild opiates, codeine combinations and stronger opiates.
2.2. Assessment Approach
Analgesic use between two patient groups taking different paracetamol formulations (Extended-Release and Immediate Release Paracetamol) were compared to determine if clinically important differences could be demonstrated using AEDs, as well as whether classes of analgesics could be combined over a 12 month period.
Proportions of patients and the proportions of prescriptions were compared with the proportions of AEDs for each analgesic class and all analgesic classes combined. Statistical significance, as well as clinically important differences, were examined among patients, prescriptions and AEDs, in patient groups taking different paracetamol formulations (ERP and IRP). Two-sided t-tests were used to compare means using SAS [11] and a Z score was used to compare proportions with a significant p-value set at 0.05. No adjustment was made for multiple comparisons.
In large prescription claims databases, like the Australian PBS, small differences can be statistically significant, but these may not be clinically important. In addition, a relative difference greater than 10% in AED scores was considered clinically meaningful. Minimal Clinically Important Differences (MCID) are easy to interpret and applied to study data. Anchor-based methods use clinical/ subjective perception to define MCIDs and should be clearly differentiated from the use of statistical significance [12].
3. RESULTS
A total of 46,255 patients aged between 50 and 85 years were initiated on OA paracetamol in the period January 2009 to December 2010 [4]. OA patients had an average age of 69 years, 62.8% were female and patients took an average of 1.8 analgesic classes over the year. A total of 44.2% took paracetamol only, while 20.3% took 3 or more classes of analgesics [4].
The AEDs values demonstrated that OA patients received around 70% of the AEDs from paracetamol. NSAIDs and COX-2Is accounted for 20% of AEDs, while opiates and codeine combination analgesics accounted for 10% of AEDs. In this group of newly initiated patients with OA, the total AEDs were similar between the two paracetamol formulations, while ERP patients took 8 more AEDs of NSAIDs and COX-2Is while IRP patients took 7 less AEDs of codeine combinations and opiates.
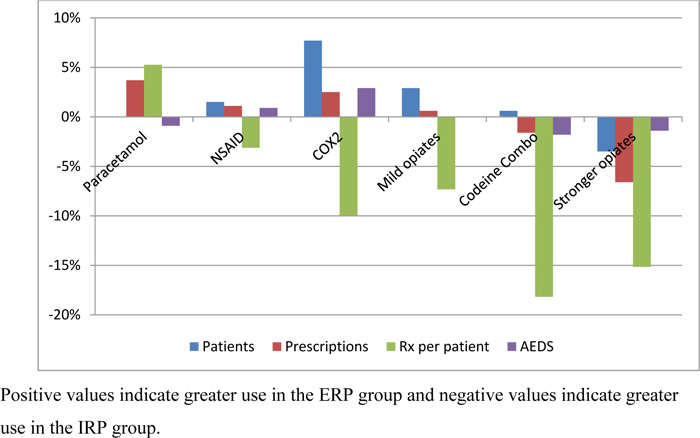
The AED values matched the majority of comparisons with the other medication use measures (Fig. 1). There was a total of 52 comparisons (18 for patients, 16 for prescriptions and 18 for prescriptions for patients. A total of 17 comparisons (65%) matched direction and 14 comparisons (54%) matched the magnitude (Table 1). These matches included: 11 for prescriptions (69%), 10 for patient numbers (56%), and 9 for prescriptions per patient (50%) (Table 1).
| - | Patients | Rxs | Rxs per Patient | |||
|---|---|---|---|---|---|---|
| - | Direction | Magnitude^ | Direction | Magnitude^ | Direction | Magnitude^ |
| Paracetamol | No | Yes | No | No | No | No |
| NSAIDs | Yes | Yes | Yes | Yes | Yes | Yes |
| COX-2I | Yes | No | Yes | Yes | No | No |
| NSAID & COX-2I | Yes | No | Yes | Yes | No | No |
| Mild opiates | No | Yes | Yes | Yes | Yes | Yes |
| Codeine Combination | No | Yes | Yes | Yes | Yes | No |
| Stronger opiates | Yes | Yes | Yes | No | Yes | No |
| All Opiates | No | Yes | Yes | No | Yes | No |
| All Analgesics | No | No | Yes | Yes | ||
| - | Patients | Prescriptions | Rx per patient | AEDs | ||||
|---|---|---|---|---|---|---|---|---|
| - | Difference^ | SS, CM | Difference^ | SS, CM | Difference# | SS, CM | Difference^ | SS, CM |
| Paracetamol | 0.0% | ns* | 3.7% | SS | 0.2 (6%) | SS | -0.9% | ns |
| NSAID | 1.5% | ns* | 1.1% | ns* | 0.0 (2%) | ns* | 0.9% | ns |
| COX-2I | 7.7% | SS, CM* | 2.5% | SS CM* | -0.5 (-10%) | SS, CM | 2.9% | SS, CM |
| NSAID & COX-2I | 9.2% | SS.CM* | 3.9% | SS CM* | -0.6 (-8%) | SS | 3.8% | SS, CM |
| Mild opiates | 2.9% | SS, CM | 0.6% | ns* | -1.0 (-22%) | SS, CM | 0.0% | ns |
| Codeine Combo | 0.6% | SS | -1.6% | ns | -0.5 (-15%) | SS, CM* | -1.6% | SS, CM |
| Stronger opiates | -3.5% | SS, CM* | -6.6% | SS, CM* | 0.0 (0.0%) | ns | -1.4% | SS, CM |
| All opiates | -1.2% | SS | -7.6% | SS, CM* | -1.5 (-9%) | SS | -3.0% | SS, CM |
| All analgesics | 7.6% | CM | NA | -1.9 (-7%) | SS | 0.0% | ns | |
Assessment of statistical significance (p<0.05) and clinically meaningful differences found: 15 (47%) were both significant and clinically meaningful, 10 (31%) were neither significant nor clinically meaningful, while 7 (22%) were significant but not clinically meaningful. No comparison was clinically meaningful and not statistically significant (Table 2).
There was coherence between AEDs and the other methods in relation to statistical significance and important clinically meaningful differences (Table 2). AEDs with COX-2I, codeine combinations and strong opiates were both statistically significant and clinically meaningful. AEDs with paracetamol, NSAIDs, mild opiates as well as all analgesics were neither statistically significant nor clinically meaningful.
4. DISCUSSION
At the time of this study, the Royal Australian College of General Practitioners (RACGP) Guidelines for the non-surgical management of hip and knee Osteoarthritis (OA) stated that: “Paracetamol has long been considered first-line therapy for OA, compared with other pharmacological options (e.g., NSAIDs, opioids).” [12, 13]
WHO guidelines recommend prompt oral administration of drugs when pain occurs, starting with non-opioid drugs. If complete pain relief is not achieved, then a mild opioid can be added to the existing non-opioid regimen. If the pain relief is not adequate, then the mild opioid can be replaced by a stronger opioid while continuing the non-opioid therapy [10]. Raffa and Pergolizzi suggested that best practice starts with non-opioid drugs at the lowest dose. If pain relief is not achieved, then a mild opioid is added to the non-opioid regime [14].
The AED methodology demonstrated that newly initiated patients received around 70% of their AEDs from paracetamol. While NSAIDs and COX-2Is accounted for 20% of AEDs, and opiates and codeine combination analgesics accounted for 10% of AEDs [4]. The total number AEDs were similar between the two analgesic formulations, while ERP patients took 8 more AEDs of NSAIDs/COX-2Is and IRP patients took 7 more AEDs of codeine combinations and opiates.
The purpose of this article was to validate the methodology for comparing the chronic use of multiple analgesics. That is, how well did the AED values match with other measures of analgesic use. In addition, how well did the AED values match the direction and magnitude of the other methods.
The number of prescriptions per patient was higher for IRP patients for all analgesic classes except for paracetamol. There were more paracetamol prescriptions in the ERP group compared with the IRP group. This was expected due to the difference in PBS approved pack size (192 vs 300 tablets).
Most of the AED values matched the other measures of analgesic use when they should, while the comparisons where the AED values did not match (Fig. 1), these were expected or could be explained. For example: The smaller pack size of ERP was expected to be offset by more ERP prescriptions being filled by ERP patients. More ERP patients taking mild opioids was offset by less prescriptions per patient for mild opioids. ERP patients taking 3% more AEDs explained by 7% more patients taking COX-2Is while ERP patients filled 0.5 less COX-2Is prescriptions per patient.
Statistically different and clinically meaningful differences were found with ERT patients who: took more COX-2I, mild opiates and less strong opiates; collected more prescriptions for paracetamol, COX-2I and less strong opiates; collected more prescriptions per patient for paracetamol and less prescriptions for all the other analgesic classes; and took more AEDs for, COX-2I and less codeine combinations, as well as less strong opiates. Neither clinically meaningful nor statistically significant differences were found with ERT patients who: took NSAIDs, dispensed prescriptions for NSAIDs, mild opioids, codeine combinations; number of prescriptions per patient for NSAIDs; and no difference in AEDs with paracetamol, NSAIDs, mild opiates as well as all analgesics.
There was coherence AEDs between both SS and CMD and with neither SS nor CMD (Table 1). AEDs with COX-2I, codeine combinations and strong were both SS & CMD; while AEDs with paracetamol, NSAIDs, mild opiates as well as all analgesics had neither SS difference nor CMD.
AED assessment was able to differentiate clinically meaningful from non-significant analgesic classes and most of these matched with one or more methods of comparing analgesic use. Where these values did not match, most could be explained.
Even though the total AED scores suggested that there was no difference in overall analgesic use between two formulations, differences in AEDs were found among the analgesic classes. The AED methodology was sufficiently sensitive to demonstrate that IRP patients climbed higher up the analgesic ladder than the ERP patients in order to obtain relief from their OA pain.
There were a number of limitations to this study. Firstly, we assumed that if a script was dispensed for a drug, then that drug was taken by the patient. Secondly, we used DDD to derive AEDs and DDDs may not reflect equal analgesic potency. Thirdly, we did not include General PBS patients because the cost of most of their analgesic prescriptions is under the patient co-payment threshold, so their details were not collected in the prescription claims database. This was a non-randomised cohort, so there may have been bias allocation in the formulation prescribed. Finally, there are no details in the DHS prescription claims database about OTC paracetamol and NSAIDs purchased from a pharmacy or supermarket.
CONCLUSION
Patients with Osteoarthritis take multiple drugs for short durations and these products have multiple strengths and different pack sizes, so it is difficult to assess the use of analgesics in mild to moderate OA pain. In order to compare analgesic treatments, a simple measure of Analgesic Equivalent Days (AEDs) was developed using defined daily doses and the total mg of each analgesic dispensed in a fixed time window.
The values from the AED method matched the majority of comparisons with the other medication use measures. Almost half of the comparisons were both significant and clinically meaningful, while one-third of comparisons were neither significant nor clinically meaningful. This indicates that the AED method is sensitive and easy to calculate. It seemed to produce valid outcomes both from a statistical and a clinically meaningful perspective.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This article describes desktop research using de-identified Australian prescriptions claims data. The Australian Department of Health’s External Request Evaluation Committee (EREC) approved this research in 2015.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data are not publicly available because EREC considers requests against privacy and secrecy considerations, and resource use.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


