All published articles of this journal are available on ScienceDirect.
Mean Platelet Volume and Red Cell Distribution Width in Differentiated Thyroid Cancer Patients
Abstract
Aim:
The purpose of this study was to investigate MPV and RDW values in DTC patients.
Background:
Differentiated Thyroid Cancer (DTC) is subdivided into papillary, follicular and papillary micro thyroid cancers. Mean Platelet Volume (MPV) and red cell distribution width (RDW) are markers which have been investigated in many cancers, but no data are available for DTC.
Objective:
MPV and RDW values were assessed in 108 patients with DTC, consisting of 44 with Papillary Thyroid Cancer (PTC) (mean age 43±13.9 years), 34 with Papillary Micro Thyroid Cancer (PmTC) (mean age 43.1 ± 10.6), and 28 with Follicular Cancer (FC) (mean age 46.9±12.5), and 77 control subjects (mean age 47.5±5.9).
Methods:
The patient and control groups were matched in terms of age, and body mass index. All subjects were investigated using platelet and biochemical parameters.
Results:
Both MPV [(PTC, PmTC, and FC) (p=0.000, p=0.000 and p=0.001, respectively)] and RDW (PTC, PmTC, and FC) (p=0.02, p=0.04 and p=0.02, respectively)] values increased in patients with DTC compared to the controls. MPV values were significantly positively correlated with CRP (r=0.247; p=0.043), postoperative thyroglobulin (r=0.246; p=0.03), gamma glutamyl transferase (r=0.024; p=0.762), tumor size (r=0.209; p=0.047) and RDW (r=0.207; p=0.005). Age, gender, total cholesterol, and C-reactive protein were identified as independent predictors of MPV. Adjustment for other these factors produced no alteration in these relative risks at multiple regression analysis.
Conclusion:
Our results suggest that patients with DTC have higher MPV and RDW values than healthy controls. MPV may represent a good follow-up criterion in DTC patients because of its positive correlation with tumor size and thyroglobulin.
1. INTRODUCTION
Thyroid cancer accounts for 95% of cancers of the endocrine system and 1.5% of all cancers worldwide. The mortality rate in endocrine cancer was approximately 66% in 2010 [1]. The disease is a heterogeneous one consisting of 80-90% Differentiated Thyroid Cancer (DTC), 5-10% undifferentiated thyroid cancer, and 5% medullary thyroid cancer. In terms of DTC subtypes, 70-80% of Papillary Thyroid Cancer (PTC) cases occur at the ages of 10-60 years, while 20-30% of Follicular Cancer (FC) occur at the ages of 25-70. Peak values are seen in the sixth decade in males and in the fourth and fifth decades in females [2-4]. The objective criterion of the World Health Organization is defined as “Incidentally detected Papillary Micro Thyroid Cancer (PmTC) smaller than 1 cm”. PmTC is a frequently seen variant of PTC. Lesions smaller than 0.2 cm can only be detected using advanced imaging techniques [2, 3]. Although patients with papillary carcinomas causing lymph node metastasis and distant metastasis have been reported, prognosis is generally good [5, 6]. The second most common variety of thyroid cancer is the follicular type, which can spread to distant areas through hematogenous dissemination. In thyroid cancer, tumor size directly affects staging, and elevated thyroglobulin levels indicate recurrence or refractory disease. Tumor staging using the size of the tumor before and three months after surgery is also important for determining the risk of DTC recurrence, classified as low, middle or high, as suggested by the American Thyroid Association (ATA) in 2015, since the TNM staging system indicates the risk of mortality. Thyroglobulin is used post-surgery for this purpose. Mean Platelet Volume (MPV) is an indicator of platelet dimensions, function and activation [7]. The correlation between MPV and many other cancer types has been investigated in previous studies [8-12]. Baldane et al. reported that MPV levels constitute an easily determined biomarker for monitoring the risk of PTC [13]. Red cell Distribution Width (RDW) increases during inflammation and indicates the variability in the dimensions of red blood cells [14, 15]. MPV and RDW have been related to conditions including chronic inflammation in cardiovascular diseases, malignancies, ulcerative colitis, hepatic cirrhosis and systemic lupus erythematosus [16-19].
Up to now, levels of MPV and RDW in the many different studies have been investigated based on between patient and control subjects. The purpose of this study was to investigate MPV and RDW values in patients with PTC, PmTC and FC.
2. MATERIALS AND METHODS
This study was carried out with the approval of the Ethics Committee of the Ataturk University Medical School (protocol number: B.30.2.ATA.0.01.00/125). We investigated 108 patients, 44 with untreated PTC (mean age 43±13.9 years), 34 with PmTC (mean age 43.1±10.6), 28 with FC (mean age 46.9±12.5), and 77 control subjects with nodular benign disease [USG and TNAB (thyroid needle aspiration biopsy) benign patients not undergoing surgery] (mean age 47.5±5.9). Total thyroidectomy was performed on all of the differentiated thyroid cancers. Radioactive iodine therapy was given for treatment PTC and FC, not for PmTC. Approval was granted by the local ethical committee. All patients were diagnosed by the endocrinology outpatient clinic. None of the subjects in this study were taking any medications affecting platelet or lipid funtions. Patients with chronic illness, panhypopituitarism hypo- or hyperthyroidism, nephrotic syndrome, a history of steroid use, diabetes mellitus, obesity, cardiac disease or using any drug known to cause hypogonadism were excluded. None of the study subjects smoked or consumed alcohol. Body Mass Index (BMI) was calculated as the ratio between weight and height squared in kg/m2. Blood samples were collected before surgery. Thyroid hormones, thyroglobulin, TPO, and antithyroglobulin (Antitg) were determined by Abbott Architect i2000 Chemiluminescence Microparticle Immunoassay (CMIA). Blood specimens were collected again after six months for the assessment of thyroglobulin and antithyroglobulin values following levothyroxine therapy. Following 12-h overnight fasting, 10 mL of blood was drawn by venipuncture into heparinized glass tubes. MPV and RDW were measured from blood specimens in EDTA. Complete test parameters were analyzed with biochemical and hematology autoanalysers using commercial kits (Roche and Beckman Coulter). The neck USG was done in sixth months by radiological physician and pathologic LAP and metastatic symptoms were investigated.
Statistical analysis was performed on SPSS 19.0 software (IBM-SPSS, Chicago, USA). Comparisons between groups were performed using Student’s t-test or the Mann-Whitney U test for continuous data. One-tailed Pearson’s correlation analysis or Spearman correlation analysis was used to determine the relations between variables. Logistic regression analysis was performed as appropriate. The chi-square test, chi-square with Yates correction and Fisher’s exact test, as applicable, were used to test associations between two findings. ROC curve analysis was used to assess the cut-off MPV with the best diagnostic accuracy for detecting DTC. P values <0.05 were considered statistically significant.
3. RESULTS
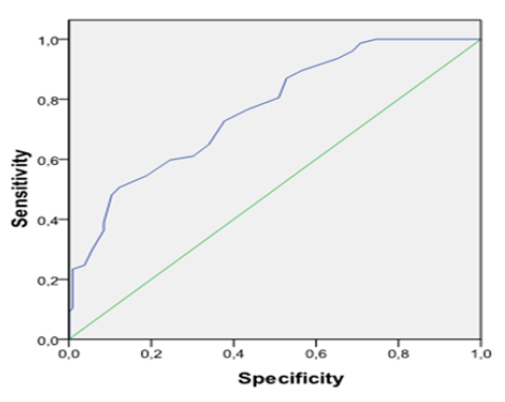
The parameter values and characteristics of the study group are shown in Table 1. The DTC group consisted of 106 patients, and the control group of 77 subjects similar to the DTC group in terms of age, and BMI distribution [PTC:43±13.9 years, 28.3±4.32 (kg/m2); PmTC: 43.1±10.6 years, 27.8 ±5.31 (kg/m2); FC:46.9±12.5 years, 27.18±4.83 (kg/m2); control: 47.5±5.9 years, 25.17±6.33 (kg/m2)]. Preoperative serum MPV values were increased in the PTC (8.87±0.99; p=0.000), PmTC (8.82±1.39; p=0.000) and FC (8.69±0.76; p=0.001) patients compared to the control group (7.82±0.82). As shown in Fig. 1, MPV values in DTC patients were higher than in the control individuals. MPV values were similar between the three DTC groups. Significant decreases in MPV levels were seen after surgery in the DTC patients (PTC=7.47±0,85, p=0.000; PmTC=7.29±1.44, p=0.000; FC=7.30±0.94, p=0.000; C=7.63±1.17, p=0.09) (Fig. 2). Serum RDW values increased in the PTC [13.7(11.6-26.8); p=0.02], PmTC [13.7(11.9-19.6); p=0.048] and FC [13.7(12.6-18.3); p=0.045] patients compared to the control group [13.2(10.46-19.2)]. Table 2 shows that preoperative MPV values were significantly positively correlated with CRP (r=0.247; p=0.043), postoperative thyroglobulin (r=0.246; p=0.03), antithyroglobulin (r=0.282; p=0.01) and RDW (r=0.207; p=0.005). The results of multiple regression analysis of MPV and other risk factors are shown in Table 3. TPO (β=0,021; p=0,257), Antitg (β=0,004; p=0,865) age (ß=-0.002; p=0.947), gender (ß=-0.399; p=0.577), total cholesterol (ß=0.001; p=0.847), and CRP (ß=0.007; p=0.898) were identified as independent predictors of MPV. Adjustment for other these factors produced no alteration in these relative risks. Other platelet counts and biochemical parameters were similar between the patient and control groups. The ROC curve for MPV predicting DTC is shown in Fig. 3. Area under the curve (AUC) was 0.76 (p=0.000). The cut-off point for MPV was >8.4 fL (sensitivity=75.3; specificity=59). There was no pathologic LAP and metastatic sympthoms in the neck USG for DTC patients.
| n | PTC | PmTC | FC | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 44 | 34 | 28 | 77 | |||||||||
| Gender (F/M) | 27/17† | 31/3† | 24/4 | 54/23 | ||||||||
| Age (yr) BMI (kg/m2) |
43±13.9 28.3±4.32 |
43.1±10.6 27.8±5.31 |
46.9±12.5 27.18±4.83 |
47.5±5.9 25.17±6.33 |
||||||||
| MPVpreop (fL) | 8.87±0.99‡ | 8.82±1.39‡ | 8.69±0.76‡ | 7.82±0.82‡ | ||||||||
| MPVpostop (fL) | 7.47±0.85 | 7.29±1.44 | 7.30±0.94 | 7.63±1.17 | ||||||||
| WBC (103/μ) | 8.72±3.04 | 8.53±2.56 | 7.97±2.95 | 8.21±2.75 | ||||||||
| Plt (x103/μL) | 264 ± 66.8 | 251.5 ± 60.2 | 261.4 ± 60.7 | 283.7±68.6 | ||||||||
| Median RDW count | 13.7(11.6-26.8)‡ | 13.7(11.9-19.6)‡ | 13.7(12.6-18.3)‡ | 13.2(10.46-19.2)‡ | ||||||||
| MCV (fL) | 83.57±10 | 84.9±6.14 | 85±6.04 | 84.7±6.8 | ||||||||
| Triglyceride(mg/dL) | 172.3±83.3‡ | 166.1± 58.3‡ | 130.1±64.9‡ | 151.4±113.9‡ | ||||||||
| Glucose (mg/dL) | 98.7±26.4 | 95.3±16.2 | 91.9±17.8 | 98.5±16.7 | ||||||||
| HDL-C(mg/dL) | 50.35±17.77 | 47.48±13.02 | 48.57±9.99 | 50.02±13.1 | ||||||||
| LDL-C(mg/dL) | 146.7±116.7 | 128.2±27.8 | 117.7±41.1 | 121.2±36.2 | ||||||||
| Total-C(mg/dL) | 202.4±55.1 | 200.7±36.4 | 192.7±46.9 | 193.3±44.4 | ||||||||
| ALT (U/L) | 24.9±12.9 | 19.4±9.77 | 20.6±10.1 | 23.6±14.3 | ||||||||
| CRP(mg/dL) | 16.3±45.7 | 3.80±4.70 | 4.87±7.07 | 3.83±4.98 | ||||||||
| fT3 (ng/dL) | 3.27±0.94§ | 3.03±0.5 | 3.39±0.78§ | 2,71±0.66§ | ||||||||
| fT4 (ng/dL) | 1.27±0.33¶ | 1.36±0.3¶ | 1.18±0.24 | 1.1±0.28¶ | ||||||||
| TSHpreop (μIU/mL) | 1.47±1.31 | 1.31±1.40 | 1.43±1.19 | 1.55±1.37 | ||||||||
| TSHpostop (μIU/mL) | 0.65±0.96 ε£ | 0.42±0.06 ε¥ | 0.57±0.18£¥ | - | ||||||||
| Thyroglobulin(ng/mL) | 0.19 (0.01- 24) | 0.19 (0.19-1.92) | 0.43 (0.19-300) | 0.46 (0.0-1.18) | ||||||||
| Antitg(IU/mL) | 153.3±537.6£ | 20.36±7.53¥ | 124.9±274.9£¥ | 55.6±89.7 | ||||||||
| TPO (ng/mL) | 132.4±295.7 | 178.9±386.5 | 32.6±44.7 | 18.6±16.6 | ||||||||
| Tumor size(mm) | 2.54±1.28 £ †ε | 0.40±0.27†¥εΩ | 2.49±1.36£¥Ω | 2.81±2.14Ω | ||||||||
†p<0.05 for PmTC patients compared with PTC, ‡p<0.05 for PTC, PmTC, FC patients compared with controls, §p<0.05 for PTC, FC patients compared with controls, ¶p<0.05 for PTC, PmTC patients compared with controls,£p<0.05 for FC patients compared with PTC, ¥p<0.05 for FC patients compared with PmTc, ε p<0.05 for PmTC patients compared with PTC, Ωp<0.05 for PmTC, FC patients compared with controls.
4. DISCUSSION
This is the first study to determine significantly high MPV & RDW values in patients with DTC by comparing PTC, PmTC and FC with a control group. The MPV cut-off point in our study was >8.4 fL, eliciting sensitivity of 75.3% and specificity of 59%. The MPV cut-off point in papillary thyroid carcinoma reported in another study was >7.8 fL, associated with 60% sensitivity and 80% specificity [14]. The sensitivity of MPV in our study was higher than that reported by Baldane et al.
MPV indicates platelet activity. Larger platelets exhibit greater reactivity than smaller platelets [20]. The pathogenesis of several forms of cancer is associated with inflammation and infection. Inflammation is a particularly crucial factor in the development and clinical course of cancer [21]. Inflammation has been reported to exhibit a number of biological effects, such as increased cellular proliferation, angiogenesis, suppression of apoptosis, and impaired adaptation to oxidative stress [22]. Platelets are implicated in the pathogenesis of cancer as a result of their inflammation-related angiogenic, metastatic, and proteolytic properties [23]. MPV reflects platelet activity and is also regarded as an important biological variable. Larger platelets have been shown to exhibit greater metabolic and enzymatic activity than smaller platelets [20]. While previous studies have examined the associations between numerous types of cancer and MPV [8-12], there has been no previous investigation of the relation between PTC and MPV. Research reporting higher preoperative MPV values compared with healthy controls includes recent studies by Kemal et al. involving ovarian cancer [10], studies by Oge et al. involving endometrial cancer [9], and two studies by Kilincalp et al. investigating colorectal cancer [11], and gastric cancer [12]. Significant reductions in MPV values have been observed following surgical tumor excision. Postoperative MPV values were also statistically significantly lower than the pre-operative values in the present study. Previous studies have investigated the association between MPV and cancer in a broad spectrum of cancers [13, 24]. Higher MPV values were reported in one study in patients with thyroid papillary carcinomas compared to subjects with benign goiter and healthy controls. MPV values also decreased after surgery [13]. Significant positive correlations were observed in this study between MPV and DTC, thyroglobulin, tumor size, CRP, RDW and total cholesterol. MPV has also been described as a potential inflammatory marker in various chronic diseases [25-27]. Ours is the first study to demonstrate positive correlations between MPV and tumor size, thyroglobulin and CRP. This suggests that MPV may have the potential for use as a marker in the monitoring of thyroid cancer. Multiple regression analysis revealed significant MPV elevation in DTC independently of age, sex, CRP and total cholesterol.
| Variables | Correlation Coefficient |
P value | |
|---|---|---|---|
| Age | -0.032 | 0.672 | |
| CRP | 0.247 | 0.043* | |
| BMI | 0.127 | 0.367 | |
| Thyroglobuline | 0.246 | 0.03* | |
| Antitg | 0.282 | 0.01* | |
| Total cholesterol | 0.037 | 0.655 | |
| GGT | 0.024 | 0.762 | |
| Tumor sıze | 0.209 | 0.047* | |
| RDW | 0.207 | 0.005* | |
* p<0.05 significantly.
| – | ß | P value | |
|---|---|---|---|
| MPV | 0.935 | 0.035* | |
| Age | -0.002 | 0.947 | |
| Gender | -0.399 | 0.577 | |
| Total cholesterol | 0.001 | 0.847 | |
| CRP mg/dL | 0.007 | 0.898 | |
| TPO | 0.021 | 0.257 | |
| Antitg | 0.004 | 0.865 | |
* p<0.05 significantly.
Inflammation may affect MPV in inflammatory diseases. Ekiz et al. reported increases in the Erythrocyte Sedimentation Rate (ESR) and CRP levels during the active stages of inflammatory diseases [28]. However, no correlation was observed between MPV and inflammatory markers. The present study is a primary report of significant correlations between MPV values and inflammatory markers such as CRP, RDW, tumor size and thyroglobulin in DTC. Malignant cells release cytokines, such as IL-1, and IL-6, and other growth factors, which stimulate platelet production. There is increasing evidence that tumors and endothelial cells are affected by Vascular Endothelial Growth Factor (VEGF), growth factors, and interleukins released by platelets [29]. This may also be involved in the transport of VEGF and platelet-derived Growth Factor (PDGF), representing powerful mitogens for a range of diverse cell types [29, 30]. These anti-angiogenic agents that specifically target these pathways have recently become increasingly used in the treatment of cancer. These agents can also affect platelet activity, which can be calculated on the basis of MPV values. If platelets do play a significant role in tumor angiogenesis, then MPV, an indicator of thrombocytic activity, may also be regarded as a potential marker of such angiogenesis. Mutlu et al. investigated 74 patients with metastatic colonic cancer and reported a significant decrease in MPV levels following treatment with the anti-angiogenic agent bevacizumab [31]. In a study of 148 patients with colorectal cancer, Tuncel et al. reported higher MPV values in patients with metastatic disease than in subjects in the non-metastatic group. Other findings determined in that study included shorter progression-free survival and a poorer response to treatment among patients with higher MPV values in receipt of bevacizumab therapy [32]. The results of that study suggest that increased platelet activity may compromise the efficacy of anti-angiogenic medications. Anti-angiogenic drug use, and particularly sorafenib therapy, has been the subject of considerable recent research in the context of advanced PTC [33]. Our study brings a different perspective on the significance of MPV and RDW in DTCs. Further and comprehensive studies evaluating the correlation between anti-angiogenic treatment and MPV and RDW in DTC patients may be required. Recent studies have demonstrated that RDW is associated with prognosis in several cancers, such as lung cancer [34] and prostate cancer [35]. RDW values in our study were significantly higher in subjects with DTC than in the control group.
CONCLUSION
In conclusion, MPV is an inexpensive method of correlating clinical activity such as thyroglobulin, tumor size and CRP with DTC. In the future, MPV may be used as a disease-activity marker in DTC. The presence of positive thyroglobulin antibodies means that there are some limitations to the use of thyroglobulin in monitoring patients with DTC. Serum thyroglobulin concentrations are used as a tumor marker in monitoring DTC. These should be determined together with Antitg at 6-12-month intervals, depending on the patient’s risk level during follow-up. Increased thyroglobulin values can be valuable in monitoring patients with DTC due to the limitations involved with positive Antitg values. Follow-up neck ultrasonography and whole-body screening can be used together since thyroglobulin cannot be employed with confidence in Antitg positivity. CT, MRI, and 18 FDG-PET/CT can be used if metastasis is suspected [36].Our findings suggest that MPV may be used as a potential biomarker in the diagnosis of patients with DTC. Since MPV can predict thyroglobulin and tumor size, changes in MPV levels can be used as an easily available biomarker for monitoring the risk of DTC in patients with thyroid nodules, thus permitting early diagnosis of DTC. In addition, a postoperative decrease in MPV levels may also be useful in evaluating therapeutic effectiveness in patients with DTC. Further studies are needed for standardization and disponibility of these parameters in patients with DTC.


ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been carried out with the approval of the Ethics Committee of the Ataturk University Medical School, Turkey (protocol number: B.30.2.ATA.0.01.00/125).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written and informed consent was obtained from each participant prior to the data collection.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


