All published articles of this journal are available on ScienceDirect.
Profiling Clinical Characteristics and Treatment Patterns Among Non-Valvular Atrial Fibrillation Patients: A Real-World Analysis in Dubai, United Arab Emirates
Abstract
Background:
There is a dearth of real-world evidence regarding patient characteristics, Oral Anti-Coagulant (OAC) treatment, and International Normalized Ratio (INR) patterns in Dubai, United Arab Emirates (UAE).
Methods:
This was a retrospective observational study among newly diagnosed adult Non-valvular Atrial Fibrillation (NVAF) patients in the Dubai Real World Claims Database. Selected patients had at least one activity claim during the 12 months pre-index date (baseline period), and a pharmacy claim for apixaban, dabigatran, rivaroxaban, or warfarin from 01 JAN 2015-31 JUL 2017. Patients with valvular heart disease, cardiac surgery, venous thromboembolism, transient atrial fibrillation, pregnancy, or OAC claims during baseline were excluded. Comorbidities and treatment patterns related to OAC use, index dosing, baseline medications, and INR patterns were described.
Results:
Among 5,072 NVAF patients, 468 met the study criteria. A minority of them (14.3%) were prescribed warfarin, and the most frequently prescribed non-vitamin K antagonist OACs (NOACs) were rivaroxaban (33.3%) and apixaban (31.4%), followed by dabigatran (20.9%). Patients’ mean age was 59 years and mean CHA2DS2-VASc score was 2.3, with most frequent comorbidities of diabetes mellitus, hypertension, coronary artery disease, and peripheral vascular disease. Additionally, 51% and 33% were on statins and aspirin, respectively, while 39% were on other anticoagulant agents. A large proportion of dabigatran patients were on a lower dose (57%). INR patterns revealed 13% of rivaroxaban, 12% of apixaban, and 7% of dabigatran patients had INR claims.
Conclusion:
This study provides relevant insights into the use of OACs in real-world clinical practice settings in Dubai, UAE.
1. INTRODUCTION
Atrial Fibrillation (AF) is the most common cardiac arrhythmia affecting as many as 33 million patients, worldwide [1, 2]. The global incidence of AF is reported to be 77.5 per 100,000 person-years for males and 59.5 per 100,000 person-years for females. In the Middle East, the incidence of AF was estimated to be 73.4 cases per 100,000 person-years for males and 49.9 cases per 100,000 person-years for females [2]. The lack of epidemiological data, and the need to understand the trends in AF management in the region call for more prospective studies in this field [3, 4].
Vitamin K Antagonists (VKAs), such as warfarin, remain the standard of care globally for the prevention of stroke among Non-Valvular Atrial Fibrillation (NVAF) patients. However, warfarin use in the United Arab Emirates (UAE) has been associated with several shortcomings – specifically caused by the lack of education about warfarin use, the importance of compliance, and the necessity for frequent International Normalized Ratio (INR) monitoring [5]. Additionally, warfarin use is also limited by potential food and drug interactions [6]. Non-VKA oral anticoagulants (NOACs), such as apixaban, dabigatran, edoxaban, and rivaroxaban have recently been used in the treatment of NVAF. These NOACs are at least as effective and safe as warfarin in large clinical trials [7-10], prompting the United States Food and Drug Administration (USFDA) to approve their use in the management of NVAF. Furthermore, based on available evidence, the Saudi Health Ministry [8], and the European and the Canadian guidelines advocate the use of NOACs in the preference to warfarin [9, 10]; a recommendation not shared by the US guidelines [11]. Specifically, the Saudi Ministry of Health advocates the preference of NOACs over VKAs in high-risk patients (with CHA2DS2-VASc scores ≥1). Although NOACs are recommended over VKAs, a recent observational study reported that most patients in Saudi and UAE were on either monotherapy warfarin or warfarin ± aspirin [12]. In view of the apparent discrepancy between clinical practice and guidelines, further research on OAC use in routine clinical practice is needed.
The approval of NOACs was based on landmark trials conducted mostly among Caucasian populations. Patients in the Africa-Middle Eastern region (AFME) were under-represented in NOAC clinical trials and global registries [13], and due to poor quality of real-world datasets in the region, there is a scarcity of such evidence in the literature [14]. Further studies based on Real-World Evidence (RWE) in the AFME region are important not only to supplement global trial evidence but also to investigate treatment patterns in the region to help guide local healthcare policies.
Commercial and public medical claims datasets have been used extensively in the west to collect RWE to compare NOACs to warfarin [15-18], and more recently, to make comparisons between various NOACs [16, 17]. Based on the above gaps in the current literature, we carried out an RWE study in Dubai to evaluate demographic characteristics and OAC treatment patterns, leveraging data from the Dubai Real World Claims Database (DRWD).
2. MATERIALS AND METHODS
This was a retrospective observational analysis using NVAF patient data from the DRWD, including medical and pharmacy claims from 1st January 2014 to 31st July 2017. The DRWD is a longitudinal patient-level database of insurance claims in Dubai, mostly from the private sector, with less than 0.1% of the total claims coming from the public sector. The database comprises over 6.9 million patients who are UAE residents and have claims for treatment from a medical facility located in Dubai. The database contains information on patient demographics, diagnoses, procedures (medical, surgical, and diagnostic), prescriptions, and other related services. The medical claims are coded using International Classification of Diseases – Tenth Revision – Clinical Modification (ICD 10-CM), Current Procedural Terminology (CPT 4), and the Dubai Drug coding system for Pharmacy claims. Institutional Review Board/Independent Ethics Committee approval was not required for this retrospective cohort analysis since researchers only had access to a limited data set that excluded patient identifiers. Patient confidentiality and anonymity of data were maintained and safeguarded throughout the study.
Adult patients (≥18 years) who had at least one medical claim with an AF diagnosis and at least one pharmacy claim for apixaban, dabigatran, rivaroxaban, or warfarin during the index identification period (1st January 2014 to 31st July 2017) were selected (Suppl. Table 1 for codes). The date of the first apixaban, dabigatran, rivaroxaban, or warfarin pharmacy claim during the identification period was designated as the index date. Moreover, since continuous enrollment information could not be reliably captured in the data, we required patients to have at least one activity claim (any inpatient, outpatient, or pharmacy claim) during the 12 months prior to the index date and to be naïve to OAC treatment (i.e., patients who had the presence of any OAC in the 12-month pre-index period were excluded) [18].
Patients were excluded from the study if they had a diagnosis of valvular heart disease/mitral stenosis, venous thromboembolism, transient AF, cardiac surgery (detailed codes can be viewed in Suppl. Table 1), a pharmacy claim for an OAC during the 12 months prior to or on the index date, or pregnancy during the study period. Detailed patient selection criteria are shown in Fig. (1). Edoxaban patients were excluded from the analysis given the late entry into the market.
Primary data variables included demographic characteristics (e.g. age and sex), as well as clinical characteristics including clinical risk scores (Deyo-Charlson Comorbidity Index [CCI] score and the CHA2DS2-VASc score), prior stroke, prior bleeding (gastrointestinal, intracranial, or other), history of diabetes mellitus, hypertension, peripheral artery disease, anemia, congestive heart failure, renal disease, peripheral vascular disease, coronary arterial disease, systemic embolism, myocardial infarction and transient ischemic attack (Suppl. Table 1). Treatment pattern data points included co-medications such as statins (atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin), anti-platelet drugs (aspirin, clopidogrel, prasugrel, ticlopidine, cilostazol, abciximab, epifibatide, tirofiban, dipyridamole, ticagrelor), other anticoagulants (low molecular weight heparin such as bemiparin, certoparin, dalteparin, enoxaparin, nadroparin, parnaparin, reviparin, tinzaparin; unfractionated heparin, fondaparinux), angiotensin receptor blockers (ARBs, such as azilsartan, candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan, valsartan). Index doses for NOACs were as follows: [apixaban (standard dose: 5 mg; lower dose: 2.5 mg); dabigatran (standard dose: 150 mg; lower dose: 75 or 110 mg); rivaroxaban (standard dose: 20 mg; lower dose: 10 or 15 mg; other dose: 10 and 15 mg OR 15 and 20 mg on the same day); warfarin had variable dosing.
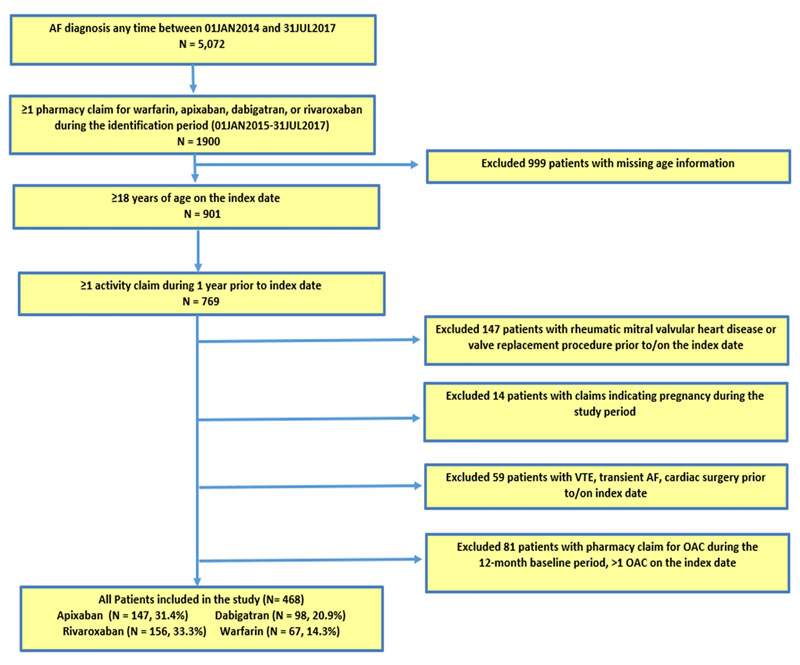
AF: Atrial Fibrillation; VTE: Venous Thromboembolism; OAC: Oral Anticoagulant.
Edoxaban patients were excluded from the analysis, given the late entry into the market.
A secondary analysis of this study was to quantify the number of INR-related claims during 180 days of follow-up (INR claims were evaluated using CPT code 85610, prothrombin time).
Descriptive analysis of clinical and demographic variables was conducted for patients prescribed NOACs or warfarin. Descriptive statistics were calculated using frequencies (n) and proportions (%) for all categorical variables, while numeric variables were summarized with means and Standard Deviations (SD). All analyses were presented for the overall population and stratified by the index OAC treatment cohort (apixaban, dabigatran, rivaroxaban, and warfarin cohorts). Due to sample size limitations, formal hypothesis testing between groups was not feasible and hence, comparisons were not conducted.
3. RESULTS
5,072 NVAF patients were identified. After all inclusion and exclusion criteria were applied, a total of 468 adult (≥ 18 years of age) NVAF patients, who had at least one pharmacy claim for either warfarin or a NOAC and had at least one activity claim in one year prior to drug use, were identified. Of these patients, 62.5% had no pharmacy claim for any OAC. Warfarin was the least prescribed medication with 67 (14.3%) patients. For NOACs, 156 (33.3%) were prescribed rivaroxaban and 147 (31.4%) were prescribed apixaban, while 98 (20.9%) were prescribed dabigatran (Fig. 1).
3.1. Demographic and Clinical Severity
The mean age of the NVAF treated patients was 59 years with warfarin patients being the oldest (mean, 61 years), followed by apixaban (60 years), rivaroxaban (59 years) and dabigatran (58 years). The majority of patients were male (71.6%). The mean CCI scores showed that warfarin patients had more comorbidities (2.3), followed by apixaban (1.6), rivaroxaban (1.2), and dabigatran (1.1) patients. Particularly, warfarin and apixaban had the highest percentage of patients with high comorbidity and risk scores. About 34-45% of the NOAC-prescribed patients had CCI scores of 0, inferring that a significant proportion of NOAC patients were generally healthy. CHA2DS2-VASc scores also indicated increased comorbidities for warfarin patients (2.8), followed by apixaban (2.5), dabigatran (2.2), and rivaroxaban (2.1); 13.5% of all patients had a CHA2DS2-VASc score=0 (Table 1).
| – | Overall Cohort | Apixaban Cohort | Dabigatran Cohort | Rivaroxaban Cohort | Warfarin Cohort | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | |
| Total Sample Size | 468 | 100.0% | 147 | 31.4% | 98 | 20.9% | 156 | 33.3% | 67 | 14.3% |
| Age | 59.1 | 14.30 | 59.5 | 14.80 | 57.9 | 13.60 | 58.6 | 14.30 | 61.3 | 13.90 |
| 18-<55 | 176 | 37.6% | 57 | 38.8% | 38 | 38.8% | 61 | 39.1% | 20 | 29.9% |
| 55-<65 | 125 | 26.7% | 38 | 25.9% | 25 | 25.5% | 43 | 27.6% | 19 | 28.4% |
| 65-<75 | 102 | 21.8% | 28 | 19.0% | 26 | 26.5% | 32 | 20.5% | 16 | 23.9% |
| ≥75 | 65 | 10.7% | 24 | 12.2% | 9 | 8.2% | 20 | 9.0% | 12 | 14.9% |
| Gender | - | - | - | - | - | - | - | - | - | - |
| Male | 335 | 71.6% | 112 | 76.2% | 72 | 73.5% | 107 | 68.6% | 44 | 65.7% |
| Female | 133 | 28.4% | 35 | 23.8% | 26 | 26.5% | 49 | 31.4% | 23 | 34.3% |
| Deyo-Charlson Comorbidity Index (CCI) | 1.4 | 1.70 | 1.6 | 1.80 | 1.1 | 1.30 | 1.2 | 1.60 | 2.3 | 1.90 |
| 0 | 173 | 37.0% | 50 | 34.0% | 40 | 40.8% | 70 | 44.9% | 13 | 19.4% |
| 1-2 | 195 | 41.7% | 63 | 42.9% | 44 | 44.9% | 61 | 39.1% | 27 | 40.3% |
| 3+ | 100 | 21.4% | 34 | 23.1% | 14 | 14.3% | 25 | 16.0% | 27 | 40.3% |
| CHA2DS2-VASc category | 2.3 | 1.70 | 2.5 | 1.70 | 2.2 | 1.60 | 2.1 | 1.70 | 2.8 | 1.80 |
| 0 | 63 | 13.5% | 13 | 8.8% | 17 | 17.3% | 28 | 17.9% | 5 | 7.5% |
| 1 | 104 | 22.2% | 38 | 25.9% | 19 | 19.4% | 36 | 23.1% | 11 | 16.4% |
| 2+ | 301 | 64.3% | 96 | 65.3% | 62 | 63.3% | 92 | 59.0% | 51 | 76.1% |
| HAS-BLED components* | - | - | - | - | - | - | - | - | - | - |
| Hypertension | 350 | 74.8% | 119 | 81.0% | 75 | 76.5% | 104 | 66.7% | 52 | 77.6% |
| Abnormal renal / liver function | 60 | 12.8% | 15 | 10.2% | 12 | 12.2% | 15 | 9.6% | 18 | 26.9% |
| Stroke | 34 | 7.3% | 8 | 5.4% | 9 | 9.2% | 9 | 5.8% | 8 | 11.9% |
| Bleeding | 81 | 17.3% | 25 | 17.0% | 9 | 9.2% | 23 | 14.7% | 24 | 35.8% |
| Labile INRs (not available) | - | 0.0% | - | 0.0% | - | 0.0% | - | 0.0% | - | 0.0% |
| Elderly (age >65 yr at index date) | 167 | 35.7% | 52 | 35.4% | 35 | 35.7% | 52 | 33.3% | 28 | 41.8% |
| Drug use | 235 | 50.2% | 82 | 55.8% | 41 | 41.8% | 66 | 42.3% | 46 | 68.7% |
| Alcohol use | - | 0.0% | - | 0.0% | - | 0.0% | - | 0.0% | - | 0.0% |
3.2. Prevalence of Comorbidities and other Risk Factors at Baseline
The most frequent comorbidities for all patients included hypertension (74.8%), diabetes mellitus (38.9%), and coronary arterial disease (32.7%). Warfarin and apixaban patients had a higher frequency of these comorbidities, followed by dabigatran and rivaroxaban. In addition, 13.9% had a history of any bleeding events (gastrointestinal, intracranial or other), wherein warfarin and apixaban patients had a higher burden. Further, 5.6% of patients had a history of ischemic stroke events, with warfarin patients being the highest burden, followed by dabigatran, apixaban and rivaroxaban patients (Table 2).
| – | Overall Cohort | Apixaban Cohort | Dabigatran Cohort | Rivaroxaban Cohort | Warfarin Cohort | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Overall Study Population | 468 | 100.0% | 147 | 31.4% | 98 | 20.9% | 156 | 33.3% | 67 | 14.3% |
| Baseline Comorbidities/Procedures | - | - | - | - | - | - | - | - | - | - |
| Diabetes Mellitus | 182 | 38.9% | 60 | 40.8% | 34 | 34.7% | 55 | 35.3% | 33 | 49.3% |
| Hypertension | 350 | 74.8% | 119 | 81.0% | 75 | 76.5% | 104 | 66.7% | 52 | 77.6% |
| Ischemic Stroke | 26 | 5.6% | 10 | 6.8% | 7 | 7.1% | 4 | 2.6% | 5 | 7.5% |
| Hemorrhagic Stroke | 2 | 0.4% | - | 0.0% | - | 0.0% | 1 | 0.6% | 1 | 1.5% |
| Bleeding (Gastrointestinal, Intracranial, Other) | 65 | 13.9% | 19 | 12.9% | 12 | 12.2% | 13 | 8.3% | 21 | 31.3% |
| Systemic Embolism | 5 | 1.1% | 2 | 1.4% | 1 | 1.0% | 2 | 1.3% | - | 0.0% |
| Cardioversion | 16 | 3.4% | 3 | 2.0% | 3 | 3.1% | 8 | 5.1% | 2 | 3.0% |
| Peripheral Artery Disease | 8 | 1.7% | 1 | 0.7% | 2 | 2.0% | 2 | 1.3% | 3 | 4.5% |
| Anemia | 55 | 11.8% | 17 | 11.6% | 7 | 7.1% | 12 | 7.7% | 19 | 28.4% |
| Congestive Heart Failure | 70 | 15.0% | 35 | 23.8% | 11 | 11.2% | 11 | 7.1% | 13 | 19.4% |
| Renal Disease | 45 | 9.6% | 13 | 8.8% | 6 | 6.1% | 8 | 5.1% | 18 | 26.9% |
| Myocardial Infarction | 16 | 3.4% | 7 | 4.8% | - | 0.0% | 5 | 3.2% | 4 | 6.0% |
| Peripheral Vascular Disease | 131 | 28.0% | 35 | 23.8% | 25 | 25.5% | 43 | 27.6% | 28 | 41.8% |
| Transient Ischemic Attack | 9 | 1.9% | 2 | 1.4% | 1 | 1.0% | 4 | 2.6% | 2 | 3.0% |
| Coronary Artery Disease | 153 | 32.7% | 44 | 29.9% | 31 | 31.6% | 48 | 30.8% | 30 | 44.8% |
3.3. Treatment Patterns
The median follow-up was shorter for apixaban (median: 7 months) compared to warfarin (median: 8 months), dabigatran (median: 10 months) and rivaroxaban (median: 10 months). This trend of follow-up time may have been driven by the market entry point of the above NOACs (i.e., apixaban approved in December 2012; rivaroxaban approved in November 2011 and dabigatran approved in October 2010 in the US)
Examination of the index NOAC dose showed that apixaban and rivaroxaban had similar proportions of patients on lower doses (~26-27%). On the other hand, the majority of dabigatran patients were seen to be on a lower dose (57%) of which, 8% were on 75 mg and 49% were on 110 mg. However, dabigatran 110 mg was the only dose available initially (Table 3).
The most frequent medication among all patients included statins (50.6%), other anticoagulants (38.5%), antiplatelet drugs (38.0%), followed by aspirin (32.9%) and ARBs (32.3%). After profiling patients for their baseline medication use, we found the most aspirin use to be among warfarin patients (40.3%), followed by apixaban (38.1%), rivaroxaban (29.5%), and dabigatran (25.5%). Among other medications, warfarin (49.3%) and apixaban patients (46.3%) were among the highest utilizers of antiplatelet drugs; warfarin (46.3%) and apixaban patients (48.3%) were among the highest utilizers of other anticoagulants while dabigatran patients most frequently used ARBs (37.8%) during the baseline period (Table 3).
3.4. International Normalized Ratio (INR) Patterns
Of the total of 468 NVAF patients, 306 were identified with 6 months of follow-up. Overall, a mean of 1.5 INR-related claims was observed among all patients with 6-months of follow-up. After examining the number of INR-related claims, we found 45 warfarin patients to have a mean of 6.1 claims over a 6-month period; 73 patients (16%) had ≥1 INR-related claim during the 6 months of follow-up, with 37% of them being warfarin patients. Interestingly, 13% of rivaroxaban, 12% of apixaban, and 7% of dabigatran patients were also found to have INR-related claims, among patients with ≥1 INR-related claim. Among NOACs a mean of approximately 4.5 INR claims was observed while a mean of 10.1 INR claims was seen for warfarin in the 6-months, among patients with ≥1 INR-related claim (Table 4).
| – | Overall Cohort | Apixaban Cohort | Dabigatran Cohort | Rivaroxaban Cohort | Warfarin Cohort | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | N/Mean | %/SD | |
| Overall Study Population | 468 | 100.0% | 147 | 31.4% | 98 | 20.9% | 156 | 33.3% | 67 | 14.3% |
| Baseline Medication Use | ||||||||||
| Antiplatelet Drugs | 178 | 38.0% | 68 | 46.3% | 26 | 26.5% | 51 | 32.7% | 33 | 49.3% |
| Other Anticoagulants | 180 | 38.5% | 71 | 48.3% | 29 | 29.6% | 49 | 31.4% | 31 | 46.3% |
| Angiotensin Receptor Blockers | 151 | 32.3% | 48 | 32.7% | 37 | 37.8% | 51 | 32.7% | 15 | 22.4% |
| Statins | 237 | 50.6% | 81 | 55.1% | 42 | 42.9% | 75 | 48.1% | 39 | 58.2% |
| Aspirin | 154 | 32.9% | 56 | 38.1% | 25 | 25.5% | 46 | 29.5% | 27 | 40.3% |
| Aspirin Dose (mean, in mg) | 102.7 | 45.50 | 99.6 | 24.10 | 104.6 | 47.10 | 97.9 | 33.80 | 115.2 | 82.00 |
| 75 mg | 30 | 19.5% | 8 | 14.3% | 7 | 28.0% | 9 | 19.6% | 6 | 22.2% |
| 81 mg | 30 | 19.5% | 11 | 19.6% | 3 | 12.0% | 10 | 21.7% | 6 | 22.2% |
| 100 mg | 112 | 72.7% | 44 | 78.6% | 18 | 72.0% | 30 | 65.2% | 20 | 74.1% |
| 300 mg | 9 | 5.8% | 3 | 5.4% | 2 | 8.0% | 2 | 4.3% | 2 | 7.4% |
| 500 mg | 1 | 0.6% | - | 0.0% | - | 0.0% | - | 0.0% | 1 | 3.7% |
| Index Dose (in mg) * | ||||||||||
| Standard dose | 262 | 65.3% | 107 | 72.8% | 42 | 42.9% | 113 | 72.4% | ||
| Lower dose | 136 | 33.9% | 40 | 27.2% | 56 | 57.1% | 40 | 25.6% | ||
| Other dose | 3 | 0.7% | 3 | 1.9% | ||||||
| Follow-up (mean, in days) | 334.2 | 263.6 | 280.4 | 240.0 | 363.2 | 271.0 | 374.6 | 278.1 | 315.8 | 250.5 |
| Median | 282.0 | 225.0 | 301.0 | 315.0 | 242.0 | |||||
4. DISCUSSION
To the best of our knowledge, this real-world observational descriptive study is one of the first real-world studies among Dubai NVAF patients treated with NOACs, which evaluated the treatment patterns, use, and clinical characteristics, leveraging data from the DRWD. Warfarin patients were older and had higher proportions of bleeding and stroke along with other comorbidities (such as myocardial infarction, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, diabetes, rheumatic and renal disease) while most who were prescribed apixaban had a history of congestive heart failure. Similar to international studies, a higher percentage of warfarin and apixaban patients had the highest comorbidity and risk scores, suggesting preferential use of these therapies for high-risk patients [18, 19]. Additionally, most dabigatran patients were found to be on lower dosing as opposed to other NOACs.
Although our current analysis was subject to loss of almost 50% of the patients due to lack of complete demographic information, the mean ages of patients were ten years younger compared to US studies [20-22] as well as European studies [21, 22]. Prior studies have found that around 30-80% of AF patients are comorbid with several severe conditions such as chronic kidney disease, obesity, coronary arterial disease, diabetes mellitus, hypertension, congestive heart failure, and hyperlipidemia, to name a few [4, 15, 23]. Our current analysis suggests a high prevalence of renal disease among warfarin users (26.9%), followed by apixaban (8.8%), dabigatran (6.1%) and rivaroxaban (5.1%); however, apixaban patients were seen to have the highest prevalence rates for hypertension (81%) and congestive heart failure (23.8%). Hence, the comorbidities add to the clinical burden in the NVAF population in this region. One of the key advantages of NOAC use is the convenience of dosing for these OACs. Bearing this in mind, apixaban and dabigatran are BID dosed, while rivaroxaban is OD dosed. With the number of comorbidities that exist in this specific population, the probable average number of other concomitant medication uses per day is quite apparent. Other studies have shown various other medication use patterns; specifically, 20-60% of NVAF patients used beta-blockers, antiplatelet drugs, cardiac drugs, diuretics, antidepressants and anti-arrhythmic drugs [24, 25]. Our study suggested that 38.0% used antiplatelet drugs and 38.5% used other anti-coagulants; of note, those prescribed NOACs were more prevalent users (32-28%) of ARBs (dabigatran patients being the most frequent users), while only 22.4% of warfarin patients used ARBs. This underscores the complexity of NVAF patients and their polypharmacy drug patterns, and therefore the choice of a once daily anticoagulant versus a more than once daily anticoagulant may be less relevant in a real-world NVAF population in terms of a potential convenience benefit [25].
| – | Overall | Apixaban Cohort | Dabigatran Cohort | Rivaroxaban Cohort | Warfarin Cohort | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N/Mean | STD/% | N/Mean | STD/% | N/Mean | STD/% | N/Mean | STD/% | N/Mean | STD/% | |
| Patients | 468 | 147 | 98 | 156 | 67 | |||||
| Number of INR-related claims* | ||||||||||
| Overall | ||||||||||
| N with 180 days of follow-up | 306 | 65% | 85 | 58% | 65 | 66% | 111 | 71% | 45 | 67% |
| Mean | 1.5 | 5.6 | 0.9 | 2.6 | 0.7 | 2.8 | 0.7 | 2.9 | 6.1 | 12.3 |
| For patients with at least 1 INR-related claim | ||||||||||
| N | 73 | 16% | 18 | 12% | 7 | 7% | 21 | 13% | 27 | 40% |
| Mean | 6.3 | 10.2 | 4.1 | 4.3 | 6.0 | 6.8 | 3.5 | 5.8 | 10.1 | 14.6 |
| Median | 2.0 | 2.0 | 3.0 | 2.0 | 3.0 | |||||
| Min | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Max | 68.0 | 17.0 | 20.0 | 28.0 | 68.0 | |||||
| 0 | 233 | 76.1% | 67 | 78.8% | 58 | 89.2% | 90 | 81.1% | 18 | 40.0% |
| 1 | 23 | 7.5% | 8 | 9.4% | 2 | 3.1% | 8 | 7.2% | 5 | 11.1% |
| 2 | 14 | 4.6% | 2 | 2.4% | 1 | 1.5% | 7 | 6.3% | 4 | 8.9% |
| 3 | 8 | 2.6% | 1 | 1.2% | 1 | 1.5% | 1 | 0.9% | 5 | 11.1% |
| 4+ | 28 | 9.2% | 7 | 8.2% | 3 | 4.6% | 5 | 4.5% | 13 | 28.9% |
| Time (in days) from index date to first INR-related claim | ||||||||||
| N | 73 | 18 | 7 | 21 | 27 | |||||
| Mean | 34.9 | 47.9 | 37.3 | 58.9 | 70.7 | 70.5 | 24.4 | 32.2 | 38.8 | |
| Median | 7.0 | 1.0 | 67.0 | 1.0 | 10.0 | |||||
| Min | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Max | 178.0 | 178.0 | 162.0 | 112.0 | 117.0 | |||||
*INR-related claim was defined based on CPT code 85610 - Prothrombin Time (PT)
Real-world claims analyses conducted in the US and Denmark have supported RCTs in highlighting standard- and lower-dose NOAC usage. Among patients prescribed apixaban, 5-18% were on a lower dose, while among rivaroxaban patients, 17-21% were on a lower dose; on the other hand, 8-10% of dabigatran were on a lower dose [7, 8, 26]. In addition, a Japanese observational study among NVAF patients admitted at a medical center in Tokyo suggested that around 20% of the patients were on lower-dose NOACs [27], which was a similar range as seen in the US and Denmark studies. In our current analysis, we observed patients on lower-dose apixaban and lower-dose rivaroxaban to be in the range of 26-27%, which was similar to RWE studies in other regions. However, a relatively high proportion of patients were seen to be on lower-dose dabigatran (~57%), compared to other RWE analyses.
Given the significant clinical burden of NVAF, there still seems to be resistance in the uptake of OACs. Some reasons may potentially include physician underestimation of patient stroke risk, physician overestimation of bleeding risk, and patients’ reluctance to take chronic warfarin due to inconvenient INR monitoring and food interactions [28]. Past studies have identified untreated AF patients using registry data – specifically, a study of the European population in the GLORIA-AF registry identified 4.1% of untreated newly diagnosed AF patients [29]; while an NVAF study utilizing the UK Clinical Practice Research Datalink (CPRD) data between 2012-2016 had as many as 15.2% untreated patients [30]. A prospective cohort study using the RAMQ database in Quebec, Canada identified 17.4% of patients without any antithrombotic treatment [31]. For AF patients in the Gulf SAFE registry (admitted to hospitals in Gulf Countries), 10% had no antithrombotic therapy [32]. Compared to the above estimates, our analysis showed a significantly high proportion of patients who remained untreated with OACs during the study period – out of the 5,072 AF patients identified during 2015-2017, 62.5% of the patients had no pharmacy claim for any OAC despite being diagnosed with NVAF; however, they could have been on other antithrombotic therapies such as aspirin. With the significant proportion of patients left untreated with OACs, education is warranted to improve the gap in treatment and to prevent impact on health outcomes. To demonstrate the projected impact on outcomes, a probabilistic AF disease model predicted the overall cost of AF-related stroke among untreated patients to be $4.5 billion per year [25]. Hence, the burden of untreated patients on the society at large can be substantial and our analysis is driven by the need for better uptake of OAC treatment in the region.
In our overall analysis, 13.5% of all the patients with CHA2DS2-VASc score=0 were seen to be on an OAC – which may infer that a considerable number of low-risk patients were under unnecessary OAC exposure. The use of aspirin was seen mostly in warfarin users (40.3%), followed by apixaban (38.1%), rivaroxaban (29.5%) and dabigatran (25.5%). A recent analysis leveraging data from the GLORIA-AF registry among Africa/Middle-East (consisting of Lebanon, Saudi Arabia, UAE and South Africa), demonstrated that overall 51.9% were on dabigatran and 31.8% were on aspirin. Moreover, among patients with CHA2DS2-VASc score=1, the proportion of use was similar to overall use (46% dabigatran; 26% warfarin users) [33]. Hence, in this study, with moderate risk, dabigatran uptake in the Middle-East is evident.
In addition to the comorbidity and treatment burden of NVAF patients, INR monitoring is an added burden and consequently adds to the inconvenience of pharmacotherapy in these patients. A prior study in UAE showed that among all NVAF patients treated with NOACs, they received 7 INR tests per year, and among only NOAC users, they received 3 INR tests during that year [15]. Our study showed that, on average, among NOAC patients who had ≥1 INR claim, NOAC users had 4.5 INR claims during the 180-day follow-up period, with dabigatran patients having a mean as high as 6 INR claims. Warfarin patients, expectedly, had a higher mean of 10 claims during the follow-up. Additionally, overall, patients received 6.3 INR tests during the 6-months period which was considerably higher as compared to the overall yearly mean in the prior study. The burden associated with INR monitoring has been evaluated in the past, inferring significant economic burden for AF patients and the healthcare system [28, 34, 35]. Hence, the INR monitoring burden for warfarin, in addition to the unnecessary burden on NOACs users, is apparent in this analysis. Additional education may need to be reinforced among key opinion leaders and physicians regarding eliminating the need for INR monitoring among NOAC users. However, our analysis only could infer the above based on a number of INR claims, as exact lab results were not available in the data.
This study has some limitations that should be considered. First, this is a retrospective observational study; therefore, causal inference cannot be evaluated. Since the number of OAC users among the identified NVAF patients were very few, we did not have sufficient power to carry out comparative analysis across OAC cohorts. Second, variables were based on ICD-10-CM diagnosis and procedure, Healthcare Common Procedure Coding System, and National Drug Codes on billing claims; therefore, coding errors and lack of clinical accuracy may have introduced bias in the study. Next, laboratory values were restricted and had limited parameters, so clinical parameters such as creatinine clearance level, international normalized ratio values and quality of anticoagulation management, body mass index, and left ventricular ejection fraction information were not evaluated. Finally, severity index, specifically mean HAS-BLED score, could not be calculated due to lack of availability of complete information for alcohol (presence or absence of alcohol use is unavailable/unreliably coded in some international data sources like US claims data [35]), INR, and demographics (such as age: due to missing age information on ~1,000 patients, these patients were excluded from the analysis); however, we highlight the prevalence of other HAS-BLED components in this analysis. Finally, this study was a representation of the quality of care in the private sector and cannot be generalizable to the public healthcare system in the UAE.
CONCLUSION
Our current RWE analysis, leveraged from the DRWD, showed that a significant majority of NOAC users were prescribed rivaroxaban, followed by apixaban, and dabigatran. The analysis suggested that NVAF patients in this region had multiple comorbidities such as diabetes mellitus, hypertension, coronary artery disease, any bleeding, and ischemic stroke. Most warfarin patients were aspirin users, while both warfarin and apixaban had other anticoagulants as well. Lastly, all NOAC patients were observed to have INR-related claims, averaging 4.5 claims over a 6-month period, suggesting an unnecessary and avoidable clinical and financial burden on this population.
Findings from this observational analysis provide important insights on the profiles and treatment patterns of NVAF patients in a real-world setting in Dubai. These insights may be utilized to understand some key areas for improvement in the treatment of NVAF patients in Dubai. One of the key areas of improvement relates to physicians’ education regarding coagulation assessment for NOACs versus warfarin. Improvements in this area might, in turn, result in more efficacious use of oral anticoagulants and more efficient use of the health resources [28]. Future research is warranted to design comparative effectiveness studies leveraging data from medical claims data to support real-world analysis done globally.
LIST OF ABBREVIATIONS
| AF | = Atrial Fibrillation |
| AFME | = Africa Middle East Region |
| ARB | = Angiotensin Receptor Blockers |
| CCI | = Charlson Comorbidity Index |
| CHA2DS2-VASc | = Congestive Heart Failure, Hypertension, Age ≥75 Years, Diabetes Mellitus, Prior Stroke or TIA or Thromboembolism |
| CPRD | = Clinical Practice Research Datalink |
| CPT-4 | = Current Procedural Terminology |
| DRWD | = Dubai Real World Claims Database |
| HAS-BLED | = Hypertension, Abnormal Renal and Liver Function, Stroke, Bleeding, Labile INR, Elderly, Drugs or Alcohol |
| ICD-10-CM | = International Classification of Diseases – 10th Revision – Clinical Modification |
| INR | = International Normalized Ratio |
| NVAF | = Non-Valvular Atrial Fibrillation |
| OAC | = Oral Anticoagulants |
| PT | = Prothrombin Time |
| RWE | = Real-World Evidence |
| SD | = Standard Deviation |
| TE | = Thromboembolism |
| TIA | = Transient Ischemic Attack |
| UAE | = United Arab Emirates |
| USFDA | = United States Food and Drug Administration |
| VKA | = Vitamin K Antagonist |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/ humans were used for the studies that are basis of this research.
CONSENT FOR PUBLICATION
Patient confidentiality and anonymity of data were maintained and safeguarded throughout the study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is derived from Dubai e-Claims database.
FUNDING
This study was funded by Pfizer Inc.
CONFLICT OF INTEREST
M Di Fusco, J Mardekian, S Kherraf, A Ghorab and N Kakoun are paid employees of Pfizer Inc, with ownership of stocks in Pfizer Inc.
R Mohamed, N Awad, A Natarajan and P Pathak are employees of IQVIA who were paid consultants to Pfizer in connection with the development of this manuscript
ACKNOWLEDGEMENTS
Editorial support was provided by Neel Vaidya and Michael Moriarty of STATinMED Research Inc.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.


