All published articles of this journal are available on ScienceDirect.
Oncoplastic Breast Surgery: A Review of Techniques Quadrant Per Quadrant
Abstract
Breast Conserving Surgery (BCS) has gradually substituted mastectomy in the treatment of early-stage tumors. Indeed it ensures the same overall survival and better aesthetic results when followed post-operative radiotherapy. Nevertheless more than 20% excision of breast tissue, retro-areolar or lower pole cancer, and higer-sized breasts with ptosis, tend to result in aesthetically unpleasant results. Oncoplastic breast surgery finds its route into breast conserving surgery in the attempt to improve the aesthetic results while not compromising the oncologic ones.
1. INTRODUCTION
Breast Conserving Surgery (BCS) has gradually substituted mastectomy in the treatment of early-stage tumors. In association with radiation therapy, it ensures patients the same overall survival with an acceptable risk of local recurrence and better aesthetic results [1]. We usually make use of quadrantectomy in BCS to reduce the amount of tissue removed, to achieve complete removal of cancer, and to preserve breast shape [2].
The original description of quadrantectomy by Veronesi involved removal of a wide portion of skin and glandular parenchyma; the technique has gradually evolved into a more conservative procedure with reduced skin and glandular resection and a skin excision pattern oriented along the lines of tension, according to Langer and Kraissl [3, 4]. However, the standard technique is not associated with acceptable cosmetic outcomes when used with certain cancer sites (lower quadrant, upper pole, retroareolar quadrant). In addition, the volume of excised glandular parenchyma is an important predictor of breast deformity occurrence [5]. Indeed, a substantial risk of deformity is reported in the literature for cases in which more than 20% of the gland is removed [6]. In these cases, oncoplastic surgery consists of wide lumpectomy with filling of the lost tissue using different plastic surgery techniques. The goal is to improve the aesthetic results and greatly increase the indications for conservative treatment.
2. ONCOPLASTIC SURGERY
The term oncoplastic, literally meaning “shaping the tumor”, was first used by Gabka et al. [7] to describe plastic surgery techniques that expanded the indications for conservative breast cancer treatment. As affirmed at the Milanese Consensus Conference on Breast Conservation of 2006, Oncoplastic Breast Surgery (OBS) must aim for widely excised, clear margins without compromising the cosmetic result, and should be performed during the same operative time as oncological excision [8]. In the last several years, there has been increased use of these techniques due to both increased knowledge of the ideal breast size and the use of computer programs for preoperative breast reconstruction planning, including digital procedure simulations [9-11].
3. PREOPERATIVE CONSIDERATIONS
Before conservative oncoplastic surgery, several factors should be evaluated including the volume of the mammary gland to be removed, the site of the tumor, the size and density of the breast (a dense breast can be easily mobilized with less risk of necrosis than a less dense breast with a greater adipose component), the presence and/or degree of ptosis, and individual patient risk factors (obesity, smoking, diabetes, prior radiotherapy/chemotherapy). Patients who are affected by T4 tumors, multicentric cancer, extensive malignant microcalcifications, or inflammatory cancer are not eligible for BCS. These individuals require simple mastectomy followed by immediate or later reconstruction through tissue expansion [12, 13].
According to Clough et al. a two-level classification system based on excised tissue volume and cancer location can be used to identify the appropriate surgical technique [14]. If the removed tissue is less than 20%, the level I technique should be used. This technique consists of the following steps: skin incision, cutaneous undermining (to ease cancer removal and subsequent glandular reshaping), detachment of Nipple–Areolar Complex (NAC), full thickness excision, defect closure by simple approximation of the margins, and any centralization of nipple areola complex [15]. If the removed tissue is greater than 20% or the breast is of high volume with severe ptosis, and if we are interested in the so-called hard-quadrant, we should choose a
level II technique. Level II is characterized by different oncoplastic techniques. Fig. (1a, b, c) According to the volume removed, the operated breast will be different than the contralateral. Therefore, the need for immediate or delayed contralateral symmetrization must be considered and discussed preoperatively [16].
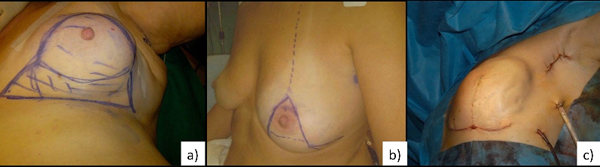
4. SURGICAL TECHNIQUES
These techniques can be broadly categorized as volume displacement, involving the principles of mobilization and transposition of local glandular (dermoglandular) flaps to fill surgical defects. Both Type I and II require volume displacement of residual glandular parenchyma, and they differ only in the volume of breast excised. For convenience in this discussion, we will divide the breast into quadrants and review the technical oncoplastic specifications for cancer in each.
5. LOWER QUADRANT (5-7 O’ CLOCK)
Several studies confirm the high risk of occurrence of major deformities in this quadrant [17-19], such as the turning down of the nipple areola complex or skin retraction. A superior pedicle mammoplasty allows oncoplastic surgeons to remove a large amount of tissue from the lower pole without causing the nipple areola complex displacement while improving the final breast shape. This technique results in inverted T- and periareolar scars [14]. The surgical technique begins with de-epithelialization of the area adjacent to the nipple-areola complex. The nipple areola complex is dissected away from the underlying breast tissue on a superior dermoglandular pedicle. The inframammary incision is then achieved, succeeded by wide undermining of the breast tissue from the pectoralis fascia. Undermining starts inferiorly and proceeds superiorly beneath the tumor, surrounding the medial and lateral aspects of the breast as well as the nipple-areola complex. The carcinoma is removed with an ample margin of normal breast tissue and overlying skin as determined by preoperative drawings. Once the resection is completed, the remodeling procedure involves opposing the two medial and lateral glandular columns in the midline to replace the defect and recentralization of the nipple areola complex [14, 20]. Lejour and Lassus described a variant to this technique by a vertical- scar mammoplasty that avoids the submammary scar [21, 22].
Additionally, Santanelli et al. proposed a variant of this technique to allow the removal of dependent tumors of the external–interior pendent quadrant [23].
6. INFERIOR– INTERNAL QUADRANT (7-9 O’ CLOCK)
In these cases, it can be useful a V-mammoplasty technique, which requires a pyramidal section of the mammary gland, with the base located in the medial portion of the inframammary layer and its apex at the areola. This technique is characterized by a wide excision of an ample section of the lower inner quadrant en bloc with overlying skin. The reshaping of the remaining mammary gland is performed by medial advancement of the residual lower quadrants after cutting the submammary fold and completely undermining the lower pole. This mammoplasty leaves a V scar formed by the radial and inframammary incisions and a periareolar scar [14, 24]
7. UPPER– INNER QUADRANT (10-11 O’CLOCK)
A BCS with abundant tissue removal may cause important alterations of the decollete. Silverstein et al. described a surgical technique that relies on the use of a preoperative type batwing design that allows the excision of large areas from this quadrant [25]. The batwing mammoplasty consists of a crescent-shaped central area of skin and gland adjoining two triangle-shape or wing-like areas of skin and gland extending from both sides of the nipple-areola complex. To perform this surgical technique, a batwing-shaped incision is drawn on the skin to surround the skin overlying the breast tumour. The lower half of the drawing should extend along the upper half of the areolar border. The upper central edge of the batwing incision will become the new superior areolar border. The glandular tissues cranial and caudal to the resection cavity are advanced together to replace the defect [26].
8. UPPER QUADRANTS (11-1 O’CLOCK)/UPPER–PARACENTRAL QUADRANTS (12 O’CLOCK)
To surgically approach these quadrants, it is advisable to use augmentation in the lower stalk with drawing/engraving preoperative according to Wise pattern excision. The preoperative drawings are equal to those described for the superior pedicle mammoplasty technique. The resection involves the upper pole. The inferior pedicle is de-epithelialized and advanced upwards towards the excision defect to achieve volume redistribution. Complementary resection is performed in the inner and outer lower quadrants to optimize breast silhouette [14] Figs. (2-9). Round block mammoplasty can also be used and will result in a periareolar scar as described by Benelli [27]. It is a simple technique where two concentric incisions are performed around the areola, followed by de- epithelialization inter-incisions skin and tumor excision. Finally, the gland is remodeled using medial and lateral glandular flaps and skin synthesis.

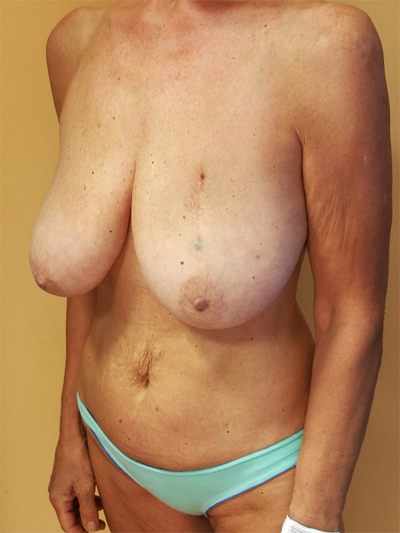


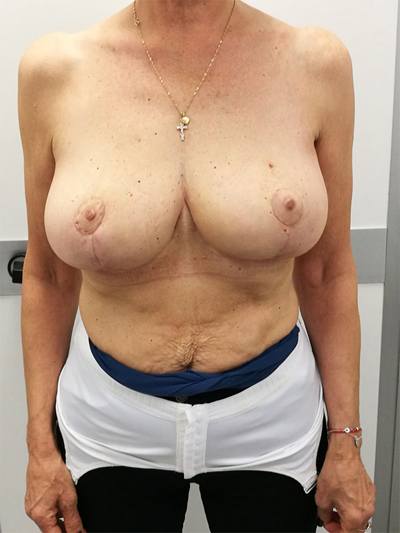
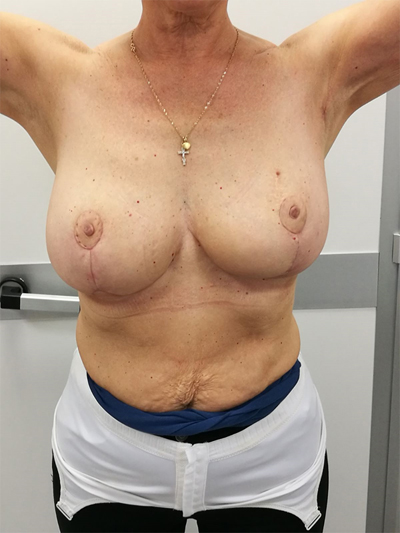
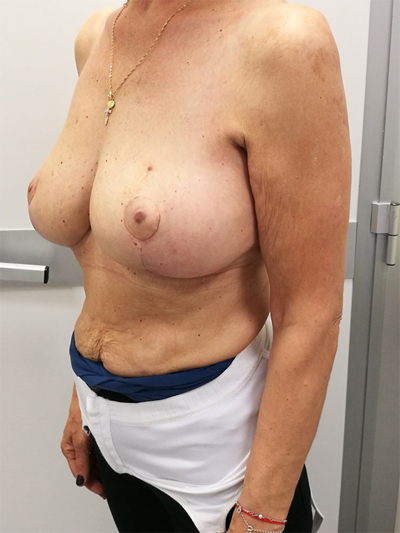
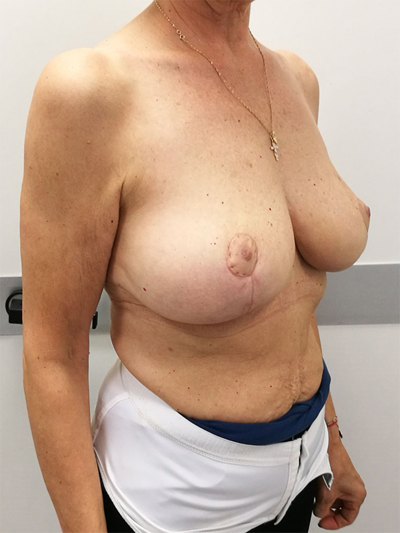
9. UPPER – OUTER QUADRANT (1–3 O’ CLOCK)
This quadrant can be described as the easiest to approach. In fact, bulky lesions may be excised using standard techniques of BCS without resulting in any breast deformity. Nevertheless, if more than 20% of breast tissue has to be removed, overlying skin may retract causing NAC migration toward ipsilateral axillary region Figs. (10-15). In this quadrant we can perform a racket mammoplasty which results in a radial cicatrix above the breast carcinoma location extending to the areola area, with repositioning of the nipple areola complex in the right site [14].
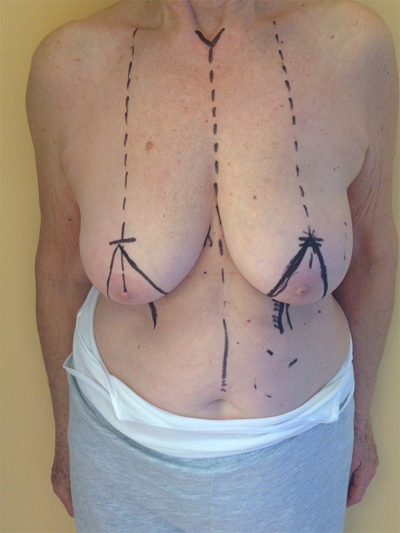
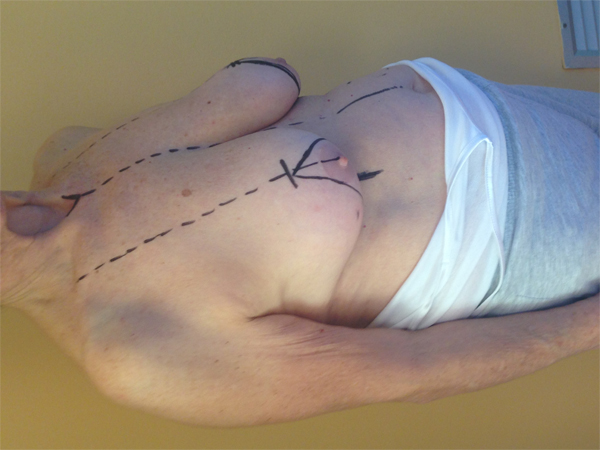
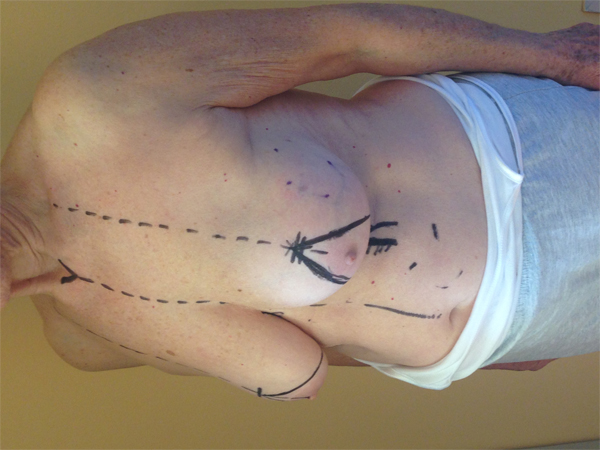
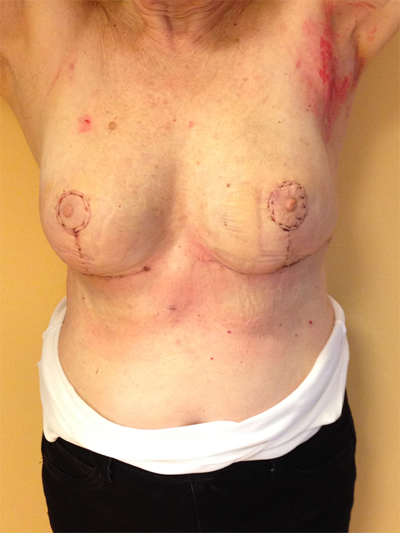
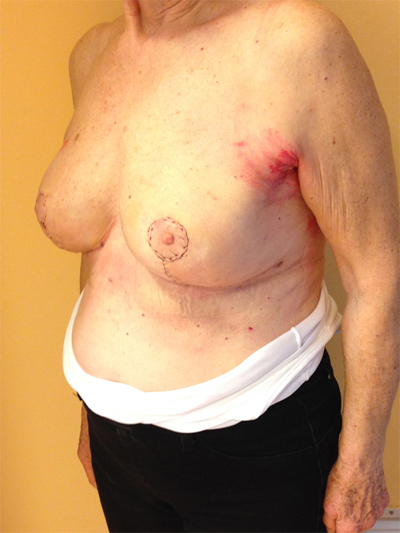
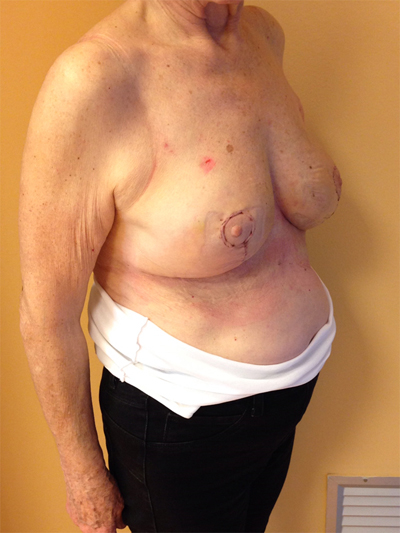
10. LOWER– OUTER QUADRANT (4–5 O’CLOCK)
The optimal procedure to approach this quadrant is a J - mammoplasty. The nipple areola complex is repositioned following the preoperative design of de-epithelialization in the upper stalk, and the mammary gland is excised in a manner which forms a letter “J”. The remaining portions of the lateral and central gland are recruited to replace the defect [28].
11. CENTRAL QUADRANTS
We proceed with excision of the NAC and breast parenchyma affected by the tumor. If the patient has a glandular breast type, allowing a large dissection, we can use the techniques described for level I. If, instead, the breast has a predominant adipose tissue composition or if resection exceeds 20% of the total volume, we use advanced techniques with round-block type reconstruction or employ a Grisotti flap [29, 30]. One possible interesting approach is to use the hemibatwing technique proposed by Pasta et al. [31]. This resection consists of a crescent-shaped area of NAC and gland, followed by a triangle-shaped or wing-like area over the adjoining skin and gland extended to one side of the areola. The surgery starts with an upper semicircular periareolar incision that is continued laterally toward the axilla. From the superior–medial side of the areola, the incision is extended to the inferior margin of the areola and then halfway around the NAC circumference. Finally, the incision is extended linearly onto the lateral external quadrant, according to the position of the lesion.
12. SECONDARY PROCEDURE
Nipple-Areola-Complex (NAC) reconstruction and Autologous Fat Grafting (AFG) can be both performed secondary to oncoplastic breast surgery techniques to ameliorate the postoperative cosmetic outcome. If the NAC has to be excised along with the breast cancer, numerous local flaps are available for its reconstruction and can be performed starting from the 2nd postoperative month; NAC tattooing has to be delayed for further 6 weeks following NAC reconstruction [32].
Residual contour deformities and asymmetry with contralateral breast can improve by the use of AFG [33]. Indeed autologous fat graft is the ideal filler by being of the same patient [34]. Furthermore, its function is more than simply filling a defect but can improve the texture of the skin, scars and any late chronic degenerative side effect of radiation therapy [35]. AFG owes its regenerative and therapeutic properties to the presence of the Adipose-derived Stem Cells (ASCs):
a population of multipotent mesenchymal stem cells that show definitive stem cell characteristics such as plastic adherence in culture, ability to maintain multipotency upon in vitro expansion, and self-renewal capacity [36-41]. ASCs can be isolated from the harvested adipose tissue by either mechanical or enzymatic means and eventually grafted together with the processed lipoaspirates [42-46]. ASCs can differentiate into multiple cell lineages and secrete paracrine factors;
hence promoting angiogenesis and wound healing, leading to higher fat graft survival as well as dermal and subcutaneous tissue regeneration [34, 42, 47-49].
Furthermore, contralateral matching surgeries can be performed at the time of the oncoplastic surgery or whenever the patient may feel requiring it [12]. Mastopexy, reductive or augmentation mastoplasties are all available techniques that every breast surgeon should be able to master easily. These procedures bring with them further potential complications (i.e., seroma, infection, suture dehiscence, flap necrosis, implant exposures, asymmetry) but are also capable of improving greatly the cosmetic outcome reducing the psychological impact that oncologic breast surgeries have on women [6, 7, 25, 50]. Therefore, contralateral matching surgeries should always be encouraged and performed at the time of the oncoplastic surgery in order to reduce the feeling of mutilation.
13. COMPLICATIONS OF ONCOPLASTIC BREAST SURGERY
According to a recent paper published by Losken et al. the complication rate with oncoplastic surgery techniques is around 16% [50]. Complications may be divided into two groups. First, there are the so-called early complications, such as delayed healing, hematoma, seroma, abscess, skin or NAC necrosis. The second group describes the late complications, including fibrosis of the scar, keloids and steatonecrosis [51]. One major complication is necrosis of the glandular breast tissue, due primarily to the mobilization of significant amounts of tissue. This it can happen especially in large-sized breast, with low gland/adipose tissure rate where breast have to be extensively dissected from the underlying muscular floor. Secondary infection of the necrotic areas may worsen the situation, resulting in increased postoperative morbidity and delayed adjuvant cancer therapies. Preoperative antibiotic prophylaxis is very important in minimizing the occurrence of these complications, and may be extended in the following day on case to case basis [52]. Furthermore, preoperative planning can benefit from new computer devices [53]. A rational approach is needed to enable surgeons to act preventively on individual risk factors (obesity, smoking, diabetes, thyroid disorders, hypertension) to lower complication rate in the postoperative period, as these factors are recognized as independently significant for the development of complications [54-56]
14. ONCOLOGICAL SAFETY
Regardless of the surgical technique used, adjuvant radiation therapy is mandatory, with several studies confirming a significant reduction in local recurrence with the advantage of better oncological results compared to the use of surgery alone [57].
A systematic review of studies of oncoplastic breast-conserving surgery demonstrated higher rates of complications with no impact on adjuvant therapies timing and oncological outcomes [58]. It has been shown that oncoplastic techniques, in addition to ensuring a more effective surgery, can provide effective radiation treatment planning. Additionally, patients with large breasts who undergo breast reductive techniques require lower doses of radiation than those treated with standard techniques [59-61].
CONCLUSION
We can then consider oncoplastic breast surgery a fundamental tool in the multidisciplinary approach to breast cancer treatment. Indeed large amount of breast tissue can be safely excised without resulting in poor cosmetic outcomes. Furthermore patient satisfaction rates highly improved while esnuring oncological safety and potentially reducing the number of re-excision and mastectomy. Possible disadvantages are long operating times and frequent need to operate on the contralateral breast in order to achieve satisfactory bilateral symmetry.
Furthermore, new prospective observational studies are needed to assess possible long-term complications of such techniques.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
There is no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


