All published articles of this journal are available on ScienceDirect.
Inhibition of Renal Fibrosis and Glomerular Injury by Sacubitril/Valsartan, a Combination Angiotensin Receptor Blocker and Neprilysin Inhibitor, in a Salt-Sensitive Hypertensive Model Using Angiotensin 1 Receptor Knockout Mice: The Contribution of Non-Angiotensin Blocking Effects to Renal Protection
Abstract
Introduction:
“Aldosterone breakthrough,” which is observed in patients receiving long term treatment with angiotensin blockade, is strongly associated with the increased risk of a declining glomerular filtration rate through the profibrotic actions of aldosterone. Sacubitril/valsartan is a newly created combination medicine (the angiotensin receptor blocker valsartan and the neprilysin-inhibitor sacubitril). Therefore, sacubitril/valsartan should have additional organ-protective actions besides the angiotensin blockade.
Methods:
In this study, we examined the renal protective effect of sacubitril/valsartan in a salt-sensitive hypertension model using angiotensin II type 1a receptor (AT1aR) knockout mice. An oral administration of 1% NaCl solution with sacubitril/valsartan (30 or 60 mg/kg/day) or valsartan (15 or 30 mg/kg/day) alone beginning 7 days before administration of aldosterone was examined in an aldosterone infusion AT1R knockout mouse model as an aldosterone breakthrough model.
Results / Conclusion:
A significant decrease in Blood Pressure (BP) was observed in the sacubitril/valsartan group compared to the valsartan group under low and high doses. In addition, the pathological analysis of the kidney for glomerular fibrosis by Sirius red staining and for injury by PAS staining demonstrated significant reductions accompanied by a significant reduction in TGF-β in the sacubitril/valsartan group compared to the valsartan group. Overall, sacubitril/valsartan, which has the dual actions of the AT1R blockade and neprilysin inhibition, may have additional clinical values for the treatment of hypertensive patients with aldosterone breakthrough.
1. INTRODUCTION
Angiotensin (Ang) II type 1 Receptor (AT1R) Blockers (ARBs) have been widely used for the treatment of hypertension and hypertension-related cardiovascular end-organ damage [1]. However, plasma aldosterone levels are known to be elevated in a subset of patients despite Ang II blockade therapy. This phenomenon, which is known as 'aldosterone breakthrough', is strongly associated with an increased risk of a declining glomerular filtration rate through the profibrotic actions of aldosterone [2]. Recently, Sacubitril/Valsartan (Sac/Val), comprising the neprilysin inhibitor (sacubitril) and the ARB valsartan, as a so-called Angiotensin Receptor-Neprilysin Inhibitor (ARNI), has been used clinically [3]. Neprilysin is a ubiquitous enzyme that is responsible for the breakdown of many vasoactive peptides, including the biologically active Natriuretic Peptides (NPs), adrenomedullin, substance P, bradykinin, ET-1, and angiotensin . Sac/Val is a crystalline compound composed of both the ARB valsartan and the neprilysin-inhibitor prodrug sacubitril, which dissociates into its component parts after ingestion. In terms of its impact on global mortality and morbidity in a heart failure (PARADIGM-HF) trial, Sac/Val significantly reduced cardiovascular and all-cause mortality, as well as Heart Failure (HF) hospitalization, in patients with HF with reduced Ejection Fraction (HFrEF) compared with the Angiotensin-Converting Enzyme Inhibitor (ACEI) enalapril [4]. Based on these clinical trials, in patients with HF, Sac/Val seems to be superior for ACEI/ARB. However, it is still unclear how Sac/Val prevented organ injury despite the Ang II blockade. To evaluate molecular mechanisms in how Sac/Val exhibited organ-protective actions, we focused on aldosterone breakthrough with long-term renin-angiotensin inhibition. To examine the effects of Sac/Val in the aldosterone breakthrough model, we employed a renal fibrosis model induced by aldosterone and 1% NaCl treatment in AT1aR-KO mice [5, 6].
Overall, in this study, we demonstrated the inhibition of renal fibrosis and glomerular injury by Sac/Val, independent from the Ang II blockade.
2. METHODS
2.1. AT1aR Deficient Mouse Model With Salt Sensitive Hypertension
Mice were divided into 6 groups: (1) nontreatment; (2) aldosterone infusion (0.15 μg/hour) + 1% NaCl treatment; (3) valsartan low dose, aldosterone infusion (0.15 μg/hour) + 1% NaCl + valsartan (15 mg/kg/day) treatment; (4) valsartan high dose, aldosterone infusion (0.15 μg/hour) + 1% NaCl and valsartan (30 mg/kg/day) treatment; (5) Sac/Val low dose, aldosterone infusion (0.15 μg/hour) + 1% NaCl + Sac/Val (30 mg/kg/day = valsartan 15 mg with NEPi 15 mg) treatment; and (6) Sac/Val high dose, aldosterone infusion (0.15 μg/hour) + 1% NaCl + Sac/Val (60 mg/kg/day = valsartan 30 mg with NEPi 30 mg) treatment (Supplement 1). Each group contained 10 mice.
We started an oral administration of 1% NaCl solution and Sac/Val or valsartan 7 days before aldosterone (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) infusion using an osmotic pump (model 1002; Alzet, DURECT Corporation, CA, USA). Aldosterone infusion was started 7 days later using eight week old AT1aR KO mice (The Jackson Laboratory), as described previously [5, 6]. Drugs were given using a flexible tube (size M mouse Pro Funnel administration system, Torpac, NJ, USA) via pharynx of mouse. Mice were housed in the animal facilities of Osaka University with free access to food and water. Mice were euthanized at 4 weeks after treatment, before which BP was measured via the tail-cuff method.
2.2. Materials
Sac/Val was donated by Novartis International AG (Basel, Swiss). Valsartan was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). All procedures were approved by the Animal Use and Care Committee of Osaka University. Systolic and diastolic BP were measured by the tail-cuff method (UR 5000: Softron, Tokyo, Japan), as described previously [7].
2.2.1. Evaluation of Renal Fibrosis and Glomerular Injury
At 35 days, the fibrosis was evaluated by Sirius red staining, and the glomeruli of the kidney were evaluated by Periodic Acid-Schiff (PAS) staining. To evaluate renal fibrosis, kidney sections (4 or 5 sections, 5 μm thick, per kidney) were stained with Sirius red staining and were analyzed visually under a light microscope by 2 investigators who were blinded to treatment. Sirius red–stained images were subsequently quantified using National Institutes of Health ImageJ [8]. To evaluate the glomerular injury, renal sections embedded in paraffin (5 μ m thick) were stained with PAS staining and examined by light microscopy. As described by [9], the glomerular injury scores were graded as 0: 0 to 10%; 1+: 10 to 25%; 2+: 26 to 50%; 3+: 51 to 75%; and 4+: 75 to 100%. They were then analyzed visually under a light microscope by 2 investigators blinded to treatment.
2.2.2. Quantitative Real-time Polymerase Chain Reaction
The total RNA was extracted from cultured cells, and quantitative real-time PCR was performed with an ABI Prism 7000 Sequence Detection System using PCR Master Mix Reagent (Applied Biosystems, Foster, USA). In each experiment, the mouse GAPDH RNA was amplified as a reference standard. Levels of mRNA were normalized to that of GAPDH mRNA, as described previously [10].
3. STATISTICAL ANALYSIS
Data are expressed as the mean ± SEM. Comparisons were made using ANOVA followed by Turkey’s simultaneous multiple comparisons. Values with P<0.05 were considered significant.
4. RESULTS
4.1. Evaluation for Renal Tubule Fibrosis by Sirius Red Staining
In this study, we compared the effects of valsartan and Sac/Val on the mouse model of salt-sensitive hypertension using AT1aR-KO mice. First, we measured the systolic and diastolic BP. There was no significant difference among all groups before pretreatment. The treatment with only 1% NaCl did not increase the systolic and diastolic BP, while treatment with valsartan as well as Sac/Val also did not decrease BP. Although the treatment with both 1% NaCl and aldosterone infusion significantly increased systolic and diastolic BP (P < 0.01), Sac/Val at a low- or high-dose treatment significantly decreased BP. The Sac/Val treatment also resulted in lower BP compared to valsartan at both low and high doses (P<0.01), (Fig. 1A, B).
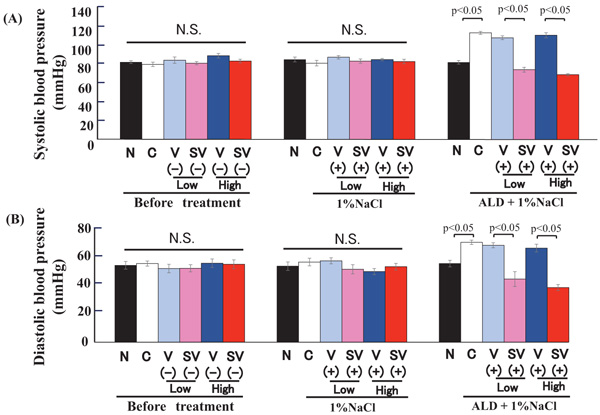
(A) The upper panel shows systolic blood pressure. Left graph; there is no significant change in systolic blood pressure before medication in AT1aR-KO mice. Middle graph; there is no significant change in systolic blood pressure after the Sac/Val (low dose (30 mg/kg/day), high dose (60 mg/kg/day)) or valsartan (low dose (15 mg/kg/day), high dose (30 mg/kg/day)) treatments. Right graph; after the treatment with aldosterone and 1 % NaCl, systolic blood pressure is significantly increased (p<0.05), and Sac/Val (low and high dose) significantly decreases the systolic blood pressure compared to valsartan (low and high dose) group (p < 0.05).
(B) The lower panel shows diastolic blood pressure. Left graph; there is no significant change in diastolic blood pressure before treatment in AT1aR-KO mice. Middle graph; there is no significant change in diastolic blood pressure after Sac/Val (low dose (30 mg/kg/day), high dose (60 mg/kg/day)) or valsartan (low dose (15 mg/kg/day), high dose (30 mg/kg/day)) treatment. Right graph; after the treatment of aldosterone and 1 % NaCl, diastolic blood pressure is significantly increased (p<0.05), and Sac/Val (low and high dose) significantly decreases the diastolic blood pressure compared to valsartan (low and high dose) group (p < 0.05). N, Non-treatment; Con, Control; V, Valsartan; SV, Sacubitril/Valsartan; Low, low dose; High, high dose; NaCl, 1 % NaCl; ALD, aldosterone.
More importantly, the Sac/Val treatment, at both low and high doses, significantly decreased renal tubule fibrosis compared to the control (P<0.01, Fig. 2), while aldosterone and the 1% NaCl treatment significantly increased the renal tubule fibrosis area as evaluated by Sirius red staining in comparison to that of the non-treatment group (P<0.01).
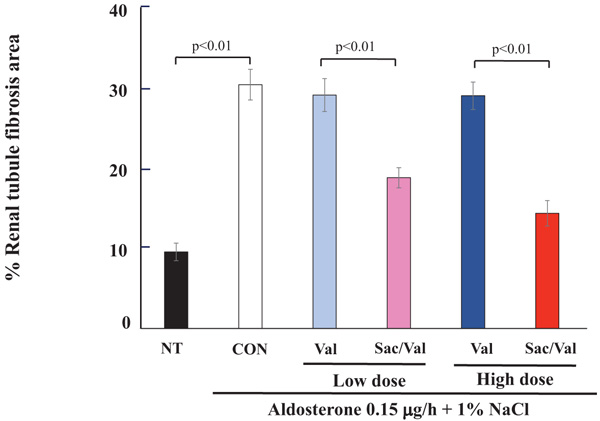
4.2. Evaluation for Glomerular Fibrosis by Sirius Red Staining and Glomerular Injury by PAS Staining
Then, we further evaluated the area of renal glomerular fibrosis. As shown in Fig. (3), aldosterone and 1% NaCl treatment significantly increased the glomerular fibrosis area as evaluated by Sirius red staining compared to the non-treatment group (P<0.01). Sac/Val treatment, both at low and high doses, significantly decreased glomerular fibrosis compared to valsartan in low- or high-dose treatments (P < 0.01, Fig. 3).
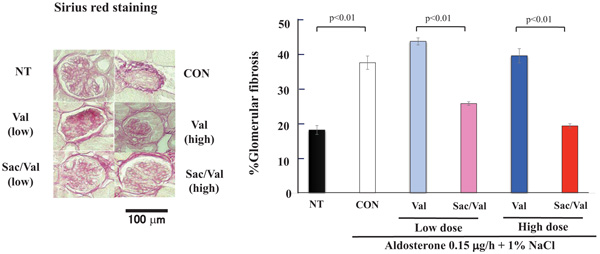
In addition, the aldosterone and 1% NaCl treatment significantly increased the glomerular injury score as evaluated by PAS staining compared to no treatment (P<0.01). Similar to other evaluations, Sac/Val treatment at both low and high doses significantly decreased glomerular injury compared to valsartan low-and high-dose treatments (P<0.01, Fig. 4).
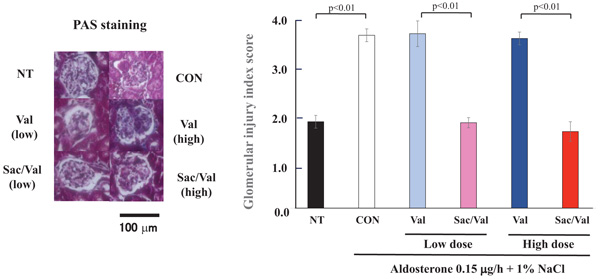
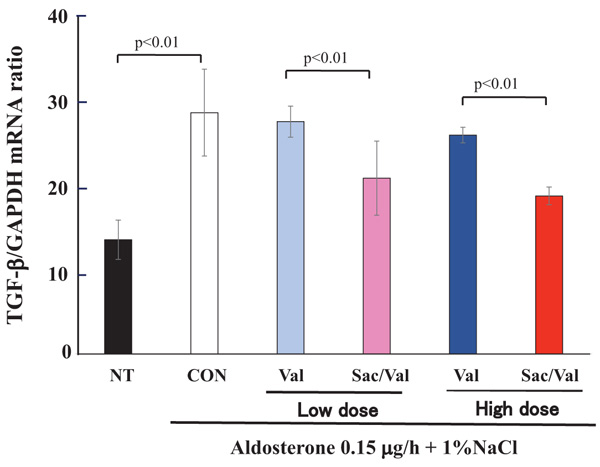
4.3. Evaluation for TGF-β1 mRNA Synthesis by Real-time PCR
Next, we further evaluated the TGF-β1 mRNA synthesis of the kidney as evaluated by real-time PCR. As shown in Fig. (5), the aldosterone and 1% NaCl treatment significantly increased the TGF-β1 mRNA synthesis of the kidney compared to no treatment (P<0.01). Sac/Val treatment, in both low and high doses, significantly decreased TGF-β1 mRNA synthesis compared to valsartan in low- or high-dose treatments (P<0.01, Fig. 5).
5. DISCUSSION
The present study showed the additional inhibitory effects of sacubitril/valsartan on renal tubule fibrosis, glomerular fibrosis and injury compared to valsartan in a salt-sensitive hypertensive mouse model using AT1aR-KO mice. In these AT1aR-KO mice, the Ang II blockade had no effects on lowering BP or on organ protection. Indeed, valsartan not only decreased the systolic and diastolic BP but also affected the tubule fibrosis, glomerular fibrosis and injury that were induced by aldosterone treatment. Nevertheless, Sac/Val treatment resulted in a significant decrease in BP and organ-protective actions on tubule fibrosis, glomerular fibrosis and injury. These data clearly demonstrated that Sac/Val likely has additional organ-protective actions, probably through the neprilysin inhibition independent from the Ang II blockade.
Did the reduction of blood pressure by Sac/Val improve renal injury? Ogawa et al. reported that hydralazine decreased the mice blood pressure induced by aldosterone and salt treatment, but it failed to improve renal fibrosis and glomerular damage [11]. The only effect of blood pressure reduction cannot improve renal fibrosis and glomerular damage in this model, so Sac/Val would improve them by the effect other than to lower the blood pressure.
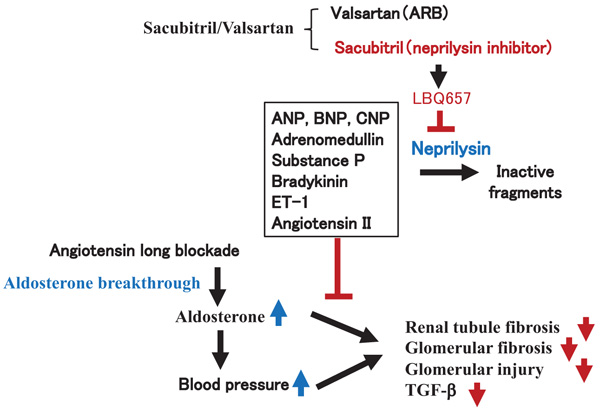
Neprilysin is a ubiquitous enzyme that is responsible for the breakdown of many vasoactive peptides, including biologically active Natriuretic Peptides (NPs), adrenomedullin, substance P, bradykinin, endothelin-1, and angiotensin (Fig. 6). NPs are endogenous protein molecules that cause urinary sodium excretion. NPs consists of the three main NPs that are secreted from the heart: Atrial Natriuretic Peptide (ANP), B-type Natriuretic Peptide (BNP), and C-type Natriuretic Peptide (CNP) [12, 13]. While CNP from endothelial cells and cardiac fibroblasts do not exert marked effects on sodium or water excretion [14], ANP primarily secreted from the atria and BNP from the ventricles are circulating hormones that counteract the volume overloaded state by promoting natriuresis, diuresis, vasodilation, and Renin-Angiotensin System (RAS) blockade [15]. ANP and BNP decrease the preload via diuresis and afterload via vasodilation and RAS blockade. CNP acts locally and exhibits vasodilatory, antifibrotic and antihypertrophic effects on the myocardium [16]. CNP inhibits deoxyribonucleic acid and collagen synthesis of cardiac fibroblast, and its effect is stronger than ANP and BNP [17]. Moreover, CNP is locally secreted in the kidney as well as the heart or vessel [18], and CNP ameliorates acute kidney injury by inhibiting apoptosis and oxidative stress [19]. Overall, circulating ANP and BNP may improve renal damage through a decrease in BP by promoting natriuresis, diuresis, and vasodilation, and local CNP in the kidney may also improve the local renal damage. An increase in NPs caused by the inhibition of neprilysin by Sac/Val might contribute to the renal protective actions observed in this study.
Another possible explanation might be the contribution of adrenomedullin and bradykinin. Adrenomedullin is a potent vasodilator that reduces systemic vascular resistance, induces renal vasodilation, increases the glomerular blood flow and filtration rate, and sodium excretion [20]. Bradykinin is also a vasodilator for coronary, celiac, superior mesenteric, renal splenic, pulmonary, gastric, and femoral artery [21] and is exerted in the blood and in the kidney [22]. These vasodilators may contribute to the decrease in BP by Sac/Val. Importantly, in the phase III clinical trial (n=1215), Sac/Val significantly decreased systolic and diastolic BP and pulse pressure compared to the use of valsartan alone to treat patients with mild-to-moderate hypertension. Sac/Val was well tolerated, since no cases of angioedema were reported [23]. Thus, the result of this study is compatible with the clinical data.
In addition, in the impact on global mortality and morbidity in heart failure (PARADIGM-HF) trial, Sac/Val reduced cardiovascular and all-cause mortality, as well as HF hospitalizations, in patients with HFrEF compared to enalapril [4], although, the addition of enalapril to conventional therapy significantly reduced mortality and hospitalization for patients with HFrEF in the SOLVD trial [24]. Then, Sac/Val was approved for the treatment of patients with HFrEF in Europe and in the USA. The current ESC-HF guideline recommends Sac/Val to replace ACEI/ARB in ambulatory patients with HFrEF who remain symptomatic despite treatment with ACEIs, beta-blockers, and mineral corticoid antagonists (MRAs) [25], while an ACCF/AHA guideline update recommends Sac/Val in patients with chronic symptomatic HFrEF and New York Heart Association class II or III who can tolerate an ACEI or ARB [26]. In addition, Sac/Val also reduced NT-proBNP to a greater extent compared to enalapril in HF patients with the preserved Ejection Fraction (HFpEF) [27]. Moreover, in the PARAMOUNT trial, Sac/Val significantly reduced NT-proBNP compared to valsartan in patients with HFpEF [28].
On the other hand, plasma aldosterone levels are known to be elevated in a subset of patients despite Ang II blockade therapy. This phenomenon, known as 'aldosterone breakthrough', is strongly associated with an increased risk of a declining glomerular filtration rate through the pro-fibrotic actions of aldosterone [2]. However, it is still unclear how Sac/Val prevented organ injury despite the Ang II blockade. To evaluate the molecular mechanisms for how Sac/Val exhibited organ-protective actions, we focused on aldosterone breakthrough with long-term renin-angiotensin inhibition. Our present study might also provide an additional organ-protective episode of Sac/Val in aldosterone breakthrough, in addition to the clinical efficacy of Sac/Val. In this study, Sac/Val improved renal tubule fibrosis, glomerular fibrosis and injury induced by 1% NaCl and aldosterone treatment compared to valsartan (Fig. 6).
In this study, we measured proteinuria in each mice, but we could not detect the difference between Sac/Val and valsartan. In addition, Jing W et al. demonstrated that there is significant renal histological and serum creatinine differences between Sac/Val and valsartan in 5/6 nephrectomy CKD rat model, but there is not a significant difference of 24h proteinuria between them [29]. Moreover, in PARAMOUT trial, the estimated Glomerular Filtration Rate (eGFR) declined less in the Sac/Val than in the valsartan group (-1.5 vs. -5.2 mL/min per 1.73 m(2) ; P = 0.002) Sac/Val is seemed to be better for renal function compared to valsartan in clinic [30]. In this experiment, we did not show the effect of renal function, but Sac/Val would improve it compared to valsartan.
In a study on the mechanism for why Sac/Val reduced renal fibrosis compared to valsartan alone, Suematsu et al. demonstrated that Sac/Val reduced cardiac fibrosis in diabetic mice through the suppression of TGF-β, but not MCP-1, IL-6, THF-a, and HIF-1a compared to the effects of valsartan [31]. The changes in those cytokines might contribute to the organ-protective actions of Sac/Val. In this study, Sac/Val treatment at both low and high doses significantly decreased TGF-β mRNA synthesis of kidney compared to valsartan in low- or high-dose treatments. The Sac/Val treatment would decrease TGF-β through neprilysin inhibition independent of an AT1R impediment. The further mechanism would be necessary in the future.
In addition, Ougaard et al. demonstrated that use of high dose losartan (30 mg/kg) but not low dose losartan (15mg/kg) decreased albumin creatinine ratio in Nephrotpxic Nephritis (NTN) mice CKD model [32]. In this experiment, there was a tendency that high dose of Sac/Val decreased more % renal tubule fibrosis area and % glomerular fibrosis area compared to low dose of that. High dose of Sac/Val may be better for reducing renal fibrosis like losartan.
CONCLUSION
The present study showed that Sac/Val exhibited more potent actions to decrease BP and inhibit renal injury and fibrosis than valsartan alone in salt sensitive hypertensive model using AT1aR-KO mice. Clearly, these additional beneficial effects of Sac/Val were due to non-angiotensin blockade, probably neprilysin inhibition. Sac/Val with the dual actions of AT1R blockade and neprilysin inhibition may provide more clinical valuable options for the treatment of hypertensive patients such as with aldosterone breakthrough. Although further studies are necessary.
LIMITATION
At first, we cannot find the molecular mechanism how neprilysin inhibitor decreases renal fibrosis and injury. At least, TGF-β1 reduction would decrease them, but we cannot conclude what decrease TGF-β1 concretely through neprilysin inhibition. Further study is needed in future. Second, we don’t check the upregulation of ANP, BNP, and CNP after Sac/Val treatment. Because the metabolism of them is too fast, we cannot detect them in the serum after drug treatment. Third, we use AT1aR KO, so AT1bR exists in mice. Valsartan (ARB) may inhibit AT1bR, but Valsartan doesn’t decrease BP in AT1aR KO miec, so AT1bR may not effect on it too much. Fourth, Telemetry System is better to measure physiological blood pressure for mice, but the system is too expensive. In this experiment, we could not use it. We used the tail-cuff method to measure mice blood pressure, but there was a significant difference between Sac/Val group and valsartan group. I believe that this result has a meaning.
LIST OF ABBREVIATIONS
| AT1aR | = Angiotensin II type 1a Receptor |
| BP | = Blood Pressure |
| Ang II | = Angiotensin II |
| ARBs | = Angiotensin II type 1 Receptor Blockers |
| Sac/Val | = Sacubitril/Valsartan |
| ARNI | = Angiotensin Receptor–Neprilysin Inhibitor |
| NPs | = Natriuretic Peptides |
| ET-1 | = Endothelin-1 |
| HF | = Heart Failure |
| HFrEF | = Heart Failure with Reduced Ejection Fraction |
| ACEI | = Angiotensin Converting Enzyme Inhibitor |
| NEPi | = Neprilysin Inhibitor |
| PAS | = Periodic Acid–Schiff |
| GAPDH | = Glyceraldehyde-3-phosphate Dehydrogenase |
| TGF-β1 | = Transforming Growth Factor Beta 1 |
| ANP | = Atrial Natriuretic Peptide |
| BNP | = B-type Natriuretic Peptide |
| CNP | = C-type Natriuretic Peptide |
| RAS | = Renin-Angiotensin System |
| HFpEF | = Heart Failure with the Preserved Ejection Fraction |
| MRAs | = Mineral Corticoid Antagonists |
| MCP-1 | = Monocyte Chemotactic Protein-1 |
| IL-6 | = Interleukin-6 |
| TNF-a | = Tumor Necrosis Factor-αlpha |
| HIF-1a | = Hypoxia-Inducible Factor-1 alpha |
| AT1bR | = Angiotensin II type 1b Receptor |
| eGFR | = Estimated Glomerular Filtration Rate |
| NTN | = Nephrotoxic Nephritis |
SOURCES OF FUNDING
This work was partially supported by a grant from the Osaka Kidney Foundation, a Grant-in-Aid from the Organization for Pharmaceutical Safety and Research, a Grant-in-Aid from the Ministry of Public Health and Welfare, a Grant-in-Aid from Japan Promotion of Science, and through special coordination funds of the Ministry of Education, Culture, Sports, Science and Technology, of the Japanese Government.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures were approved by the Animal Use and Care Committee of Osaka University.
HUMAN AND ANIMAL RIGHTS
No humans were involved in this study, the reported experiments on animals were in accordance with the standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals (http:// grants.nih.gov/grants/olaw/ Guide-for-thecare-and-use-of-laboratory-animals.pdf) published by the National Academy of Sciences.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
This study was financially supported by Novartis Pharma K.K., Japan. Sacubitril/Valsartan was provided by Novartis AG.
ACKOWLEDGEMENTS
We appreciate Miss Hitomi Sasaki for official support, and all of the members of the Clinical Gene Therapy group at the Osaka University Graduate School of Medicine, for much helpful discussion and technical support.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Web site along with the published article.


