All published articles of this journal are available on ScienceDirect.
Nipple Reconstruction Techniques: Which is the Best Choice?
Abstract
Nipple-Areolar Complex (NAC) reconstruction represents the final, concluding journey of breast reconstruction by being able to give to reconstructed breasts the shape of a natural breast mound. Nevertheless an enormous amount of nipple reconstruction techniques are described in literature, given the fact that most reconstructive options fail to give satisfactory outcomes in relation to the long-term nipple projection. In this review, the authors will browse most common nipple reconstruction techniques, taking into account: Indication, outcome, and side effect. Composite nipple grafts, traditional flaps, flaps with autologous graft augmentation, flaps with allograft augmentation, and flaps with alloplastic augmentation are the main strategies employed nowadays. Composite nipple grafts give the best guarantee of color-texture match with the contralateral side and show satisfactory nipple projection even at long-term follow-up. Skate, star, C-V, and arrow flap are by far the most commonly employed and the most reliable local flaps, however loss of projection of up to 70 percent are reported in literature. Alloplastic grafts were associated with the lowest rates of projection loss followed by autologous and allogenic ones. Nevertheless allogenic grafts are also associated with the highest complication rate, while autologous and allogenic ones have similar rates. Infection, seroma, and fat necrosis are the more commonly reported complications of autologous grafting along with donor site morbidity, while allogenic and alloplastic augmentation grafts may also experience the risk of overcorrection and graft exposure. Given the numerous techniques described in literature it is clear that the ideal nipple reconstruction hasn’t been found yet. Whereas it should be chosen on case to case basis depending on type of mastectomy, radiotherapy, type of reconstruction, skin thickness, tissue condition, and patients’ expectations to ensure the best cosmetic outcome.
1. INTRODUCTION
Nipple-Areolar Complex (NAC) reconstruction represents the final, concluding journey of breast reconstruction [1]. Despite being the less technically challenging step of breast reconstruction, it is of great concern for patients from an aesthetic and psychological point of view [2]. Indeed recreated NAC can finally give to reconstructed breasts the shape of a natural breast mound. Furthermore NAC reconstruction can be done anytime and following any type of breast reconstruction strategy.
Nevertheless an enormous amount of nipple reconstruction techniques and approach can be found in literature, given the fact that most reconstructive options fail to give satisfactory outcomes in relation to the long-term nipple projection [3].
2. MATERIALS AND METHODS
In this review, the authors will browse most common nipple reconstruction techniques, taking into account: indication, outcome, and side effect. Notes of surgical technique won’t be taken into consideration since it goes beyond the aim of this paper and are described in deep in numerous papers and books chapters.
A literature search was conducted across PubMed database from inception to March 2018 in order to identify a list of article of potential interest. Among those identified, manual review of titles, abstract and main text was conducted sequentially to capture relevant articles to be added to our paper. The keywords used for the search in the database were: “nipple,” AND “reconstruction,” OR “augmentation,” OR “areola,” OR “breast reconstruction,” OR “graft,” OR “allogeneic,” OR “autologous,” OR “synthetic”.
Prospective, retrospective studies, and systematic reviews were included only if reporting outcomes of either nipple or NAC reconstruction following mastectomies. Each articles had to report nipple reconstruction outcomes and follow-up periods in order to be included. Whereas studies only describing surgical techniques or reviews with patient cohorts of articles already included in this review were excluded Chart. (1).
3. RESULTS
3.1. Anatomical Landmark
When recreating a normal breast mound, correct anatomical landmarks should be clearly kept in mind. Ideally, the nipple should be located at the point of maximum projection on the breast mound as it can be seen in a youthful breast, having a sternal notch-nipple distance ranging from 19 to 21 cm and a nipple to inframmammary fold distance ranging from 7 to 8 cm [3].
Furthermore nipples can differ even greatly from one person to another for what concerns: Size, shape, color, and position. Nevertheless nipples have an average 1.3 cm diameter, 0.9 cm high, and nipple-areola ratio of approximately 1:3 [4].
These measures can be particularly helpful in patients that underwent bilateral breast reconstructions, while the contralateral side guides the reconstructive strategy in monolateral ones.
3.2. Timing
Adjuvant therapies and revisional reconstructive procedures (including contralateral matching surgeries) guide timing of NAC reconstruction, which should be ideally done after a 3-5 months period following the last reconstructive procedure in order to let inflammation and edema fade [3]. Furthermore the breast mound would appear in his final shape having reached the desired grade of ptosis after this time break [5].
3.3. Patient Selection
Based on the reconstructive strategy, proper nipple reconstruction technique should be employed. Indeed prosthetic-based reconstructions tend to have thin skin as a consequence of skin expansion, while autologous reconstructions have skin paddle of various shape and varying skin thickness depending on the flap used [3]. Furthermore when present, contralateral NAC characteristics have to be taken into consideration when planning adequate matching reconstruction approach.
3.4. Nipple Reconstruction Techniques
Among the many ways to recreate a nipple, reconstructive strategies can be dived into the following categories: composite nipple grafts, traditional flaps, flaps with autologous graft augmentation, flaps with allograft augmentation, and flaps with alloplastic augmentation.
4. COMPOSITE NIPPLE GRAFTS
Special consideration should be given to composite nipple grafts. Distant site composite grafts were employed successfully. Toe pulp, and labial tissue grafts gave satisfactory results; however donor site morbidity made these techniques fall in disuse [6, 7]. Only contralateral nipple grafts still be even nowadays a widespread technique when patients have a contralateral over projected nipple [8]. Indeed nipples exceeding 1 cm in high can be safely removed of their distal portion to be used as free composite graft for contralateral nipple reconstruction with aesthetically pleasant outcomes. Nevertheless contralateral surgery, additional morbidity, reduced contralateral nipple sensation, and related addition emotional/psychological distress make patients incline to different reconstructive options [8]. Contralateral composite nipple graft is a feasible option even in irradiated breasts, and graft survival is likely to occur if proper medications are performed in the postoperative days [9]. Nipple grafts usually have an early postoperative discoloration; however altered pigmentation and hypertrophic scarring are usually not observed. Indeed composite nipple grafts give the best guarantee of color-texture match with the contralateral side and show satisfactory nipple projection even at long-term follow-up [10-12] (Table 1).
5. TRADITIONAL FLAPS
Over the last 30 years numerous local flaps along with their modifications were designed for nipple reconstruction; all of them owe their design to the Little’s skate flap and Anton’s arrow flap [13-35]. The development of a high amount of flaps described in literature can be explained the lack of the currently available techniques to provide reliably and predictably projection to the reconstructed nipples over time. Indeed 25 to 50% projection are lost on average due to scar contraction, thus reconstructed nipples should be initially overprojected in order to obtain satisfying cosmetic outcomes [35].
Two are the main causes of loss of projection: retraction forces and tissue contraction. Retraction forces exerted by surrounding and underlying tissues in order to return to their original position have to ability to reduce projection [35]. Furthermore centrally-based flaps are more subjection to retraction forces than subdermal-based ones. Contraction or shrinkage of reconstructed nipples occurs as a consequence of scar contraction and compromised blood supply at various and unpredictable degree.
Among the various flaps designed, C-V, S, star, skate, and arrow flaps are by far the most commonly employed [2].
Skate flap is one of the most appreciated flaps since it can reliably recreate a projected nipple over time [3]. It’s a centrally-based flap that includes subdermal fat to guarantee long-term nipple projection. In its original design it leaves a doughnut-shaped area that needs to be grafted recreating the areola at the same time of nipple reconstruction. Modifications allowed for direct closure, nevertheless tapered flap design increases tension and retraction forces and tend to flatten reconstructed breasts.
Shestak [15] compared long-term results of reconstructed nipples with bell, skate, and star flaps and have found out that: (1) major loss of projection occurs during the first 3 post-operative months, while results tend to stabilize after the first six; (2) bell flap gives the worse results over time, while skate and arrow flaps can achieve the best outcomes; (3) skate flap is the most appropriate choice when projection goal is >5 mm. Richer et al. [16] also describes skate flap as the best reconstructive choice. Zhong et al. [17] described the high series of reconstruction (422 patients) with the skate flap, and reported a mean projection of 2.5 mm at 44 months of follow-up. Furthermore they reported that minor complication occurred in 7.22% of reconstructions: skin graft donor site dehiscence was the most common (3.1%) followed by partial graft non-take (2.1%).
Star flap has similar design to skate flap, nevertheless it allows for direct closure not requiring skin graft, thus reducing donor-site morbidity [18]. Opposing to skate flap, direct closure do not flatten reconstructed breast, furthermore the result scar is linear and can lie on the one resulting from skin sparing mastectomy. However it is reported to have lower nipple projection retention at long-term follow-up [19]. Banducci et al. [20] reported that star flap retain higher long-term projection when performed on autologous reconstructions, while Few et al. [21] reported no differences between autologous and implant-based ones. Furthermore they reported that wings lengths predictably correlate with initial and long-term projection.
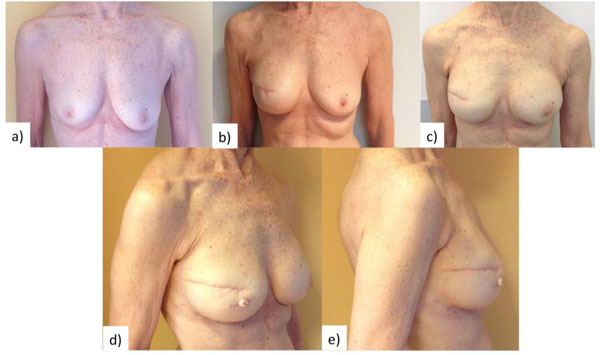
C-V flap fuses skate and star flap designed into a pedicle-based flap that is easy to elevate and ensure direct closure of donor site [15, 22-24]. When employed for nipple reconstruction in implant-based breast reconstructions, higher amount of vascularized tissue should be included in the core of the flap to compensate for the dermal thinning caused by tissue-expansion stage (Fig. 1).
Arrow flap is a versatile flap characterized by geometric design, ease of dissection, and fast learning curve [25, 26]. It allows for direct closure but it adds a further vertical scar that can be covered by tattooing of the areola. Rubino et al. [26] reported a better projection retention of their modification of the arrow flap when compared to the modified star flap. Furthermore no outcome difference was reported between autologous and implant-based breast reconstructions, with good patients satisfaction and compliance.
Bell, double opposing peri-areolar/purse-string, and top-hat flaps have different design than the previous ones since they are defined as pull-out/purse-string flaps [15, 26-29]. These flaps mobilize surrounding tissue through purse-string techniques in order to recreate a projected nipple; however a supple breast mound is required to perform these flaps.
As previously reported, Shestak [15] found that bell flap is not able to provide over time highly projected nipples, hence it should be performed only in case of contralateral nipple that has a projection of 2-3 mm.
Double opposing peri-areolar/purse-string flap is a good option in case of autologous-based reconstructed breasts that have large skin paddles and thick dermal/subcutaneous layers [27, 28].
Top-hat flap has peculiar design given the fact that no true flap is lifted, and purse-string sutures are used to give projection to the reconstructed nipple [29]. It is a centrally-based flap with broad base, thus high retraction forces are exerted preventing it from being able to achieve projected nipples over time.
Finally, S, double-opposing tab, and spiral flaps are the best choice when blood supply is an issue [18, 30-33]. Indeed, low blood supply is not only responsible for promoting scar tissue formation hence reconstructed nipple retraction, but also can led to partial or total flap loss.
S flap is a double-opposing flap oriented in a S-configuration, ideated by Cronin [33] in case of transversely located mastectomy scars. In the modification of Lossing et al. [30] no skin grafts were required for donor-site closure and 1 cm back-cuts were added to ease flap rotation, however the risk for tip necrosis increased greatly. Furthermore 80% projection was lost at 36 month of follow-up.
Double-opposing tab flap is also designed as double-opposing flaps placed on either side of mastectomy scars that transverse ideal nipple position. In the works of Kroll [18, 32] double-opposing tab flap proved itself to be a better choice than S flap by being able to provide satisfying projection over long-term follow-up.
Spiral flap is characterized by simple design and ease of elevation. It employs only little amount of breast skin since it is designed over the mastectomy scar. However including scar tissue within the flap can result in reduced blood supply and limited projection over long-term follow-up [34] (Table 2).
| Flap | Authors | N° Patients | Projection (mm) | Follow-up (months) | Final projection (mm) | Projection loss (%) | Satisfaction rate (%) |
|---|---|---|---|---|---|---|---|
| Skate flap | Shestack et al. [15] Richter et al. [16] Zhong et al. [17] |
23 29 422 |
N/A N/A N/A |
12 9 44 |
N/A 9.24 2.5 |
40.94 45 N/A |
N/A N/A N/A |
| Star flap | Shestak et al. [15] Banducci et al. [20] Few et al. [21] Kroll et al.[18] Rubino et al.[26] |
28 28 93 47 16 |
N/A N/A 10-21 N/A N/A |
12 38 12 24 12 |
NA NA 4-8.3 1.97 3.25 |
43.36 71.3 59 N/A 66.7 |
N/A N/A N/A N/A N/A |
| C-V flap | Losken et al.[22] Valdatta et al. [23] El-Ali et al. [24] |
11 29 50 |
N/A N/A N/A |
60 12 15 |
3.77 3.52 2.17 |
N/A 32 45 |
81% N/A 72% |
| Arrow flap | Li et al.[25] Rubino et al.[26] |
N/A 16 |
N/A N/A |
3 12 |
NA 4.75 |
50 50.9 |
N/A N/A |
| Bell flap | Shestak et al.[15] | 17 | N/A | 12 | NA | 73.95 | N/A |
| Peri-areola/purse-string flap | Dolmans et al.[27] Shestak et al.[28] |
14 47 |
N/A N/A |
12 12 |
5 N/A |
50 N/A |
82 N/A |
| Top-hat flap | Gamboa-Bobadilla[29] | 23 | N/A | 18 | 6 | N/A | 90 |
| S-flap | Cheng et al. [30] Lossing et al. [31] |
N/A 21 |
N/A N/A |
18 36 |
3.27 3.9 |
NA 80 |
86.4 82 |
| Double-opposing tab flap | Kroll et al. [18] Kroll & Hamilton [32] |
106 50 |
N/A 11.1 |
24 >10 |
2.43 3.8 |
NA 66 |
N/A N/A |
6. AUTOLOGOUS GRAFT WITH TRADITIONAL FLAPS
In the attempt to provide long-term maintenance of projection to reconstructed nipples, autologous, allogenic, and alloplastic grafts were incorporated in the architecture of traditional flaps [7, 36-58].
Among the various autologous grafts available, costal cartilage is one of the most valuable, particularly when performing free flap breast reconstructions where a little portion of costal cartilage is removed during dissection of the internal mammary vessels and can be banked for the time of nipple reconstruction [36-40]. Thus costal cartilage harvest has minimal donor site morbidity and authors reported sustained nipple projection. However costal cartilage graft are discouraged in case of breasts reconstructed by means of thick and stiff skin flap as the SGAP flap because of the unsatisfying results [39]. Furthermore costal cartilage guarantees superior nipple projection maintenance over time when compared to the other autologous grafts employed, however the excessive rigidity of reconstructed nipples can be an issue for patients. Finally complications as partial flap loss, nipple malposition, and cartilage exposure may occur in as high as 12% of reconstructions [39].
Auricular cartilage was also advocated as augmentation graft for nipple reconstruction [41, 42]. Results showed higher loss in projection when compared to costal cartilage grafts, with minimal but still higher donor site morbidity, and low complication rate.
Fat graft has been also proposed for reconstructed nipple augmentation. Bernard [43] advocates for fat grafting the site where nipple is going to be recreated prior to C-V flap elevation, especially in breast that underwent tissue expansion. While Eo et al. [44] grafted dermal fat harvested from discarded breast tissue at the center of a C-V flap. Fat graft is easy, fast, with minimal donor site morbidity, and they found satisfactory long-term nipple projection. However high reabsorption rate and projection loss are reported in radiated breasts.
Finally, Chia et al. [45] advocated for the use of stacked dermal grafts harvest from the dog-ear deformities of previous scars in conjunction with C-V flap. Reabsorption rate ranged from 25% to 30% and initial overcorrection should be performed. They found out that nipples reconstructed with thicker dermis of Latissimus Dorsi skin island were less prone to contracture. Furthermore patietns that underwent adjuvant radiotherapy had poorer results and higher complications (Table 3).
| Autologous Grafts | Local flap | Authors | Grafts size | N° Patients | Projection (mm) | Follow-up (months) | Final projection (mm) | Projection loss (%) | Satisfaction rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| Costal cartilage graft | Arrow flap Modified top hat C-V flap |
Guerra et al. [36] Heitland et al. [37] Cheng et al. [38, 39] Mori et al. [40] |
10 x 10 x 15 mm N/A 10 x 10 x 10 mm N/A |
454 17 58 8 |
N/A N/A 11.1 N/A |
84 12 45 12.6 |
N/A N/A 8.2 N/A |
N/A 25 26.1 41 |
N/A 76.5 N/A N/A |
| Auricular cartilage graft | C-V flap Bilobed Trilobed flap |
Jones & Erdman [41] Tanabe et al. [42] |
N/A N/A N/A |
23 8 6 |
10 N/A N/A |
24 21.3 31.6 |
3.3 7,4 3.1 |
67 5.4 61 |
57 N/A N/A |
| Fat graft | C-V flap | Bernard & Beran [43] Eo et al. [44] |
1-2 cc 1x1x2 cm |
13 20 |
N/A 12-18 |
12 12 |
N/A 6-12 |
N/A 25-50 |
N/A N/A |
| Dermal graft | C-V flap | Chia et al. [45] | N/A | 40 | 11.5 | 12 | 8 | 30 | N/A |
7. ALLOPLASTIC GRAFT AUGMENTATION
Alloplastic grafts were investigated in place of autologous one for nipple reconstruction in order to reduce morbidity related to donor sites. Alloplastic augmentation grafts proved to be able to retain long-term nipple projection in various studies [7, 46-50]. Hallock [46] in 1990 was the first to describe alloplastic grafts augmentation for nipple projection. He inserted polyurethane-coated silicon gel implants in subdermal pockets in two patients that underwent autologous-based breast reconstruction. No loss of projection was observed at long-term follow-up.
Evans et al. [47] described the use of calcium hydroxylapatite gel (Radiesse; BioForm, Inc., Franksville, WI, USA) for secondary nipple reconstructions. Patient satisfaction at 10 months follow-up was the only parameter assessed, and no postoperative complications were reported.
Panettiere et al. [7] described the use of labia minora wedge and composite nipple graft in conjunction to hydroxyethyl methacrylate and ethyl methacrylate hyaluronic acid suspension (DermaLive marketed through Dermatech, Paris, France) injections performed at the 2nd postoperative months. Good long-term nipple projection, high patient satisfaction rate and no complications were reported. However outcomes were significantly better when patients underwent nipple reconstruction with labia minora wedge and hydroxyethyl methacrylate and ethyl methacrylate hyaluronic acid suspension injections. Furthermore one patient had false-positive result on PET scan.
Yanaga [48] employed artificial bone (Ceratite; Chugai Medical Device, Tokyo, Japan) as augmentation graft in nipple reconstruction positioned in the core of elevated traditional flaps. No loss of nipple projection was reported at long-term follow-up in all patients treated.
Wong et al. [49] reported the use of polytetrafluoroethylene in nipple reconstruction. Overall patient satisfaction rate was high and good nipple projection was retained. Implant extrusion was reported in one case only that required removal and replacement.
Jankau et al. [50] perfromed 30 nipple reconstruction by means of C-V flap along with a silicone rod (NA 515-7, Nagosil; Nagor Ltd., Isle of Man, United Kingdom) for augmentation. All patients that underwent breast reconstruction by means of latissimus dorsi flap developed nipple necrosis, implant exposure and healed by secondary intention. While the remaining patients that had undergone breast reconstruction by means of TRAM flap had no complications and experienced a 33.9% decrease in nipple projection at 10-month follow-up (Table 4).
| Alloplastic Implants | Authors | N° Patients | Grafts size | Projection (mm) | Follow-up (months) | Final projection (mm) | Projection loss (%) | Satisfaction rate (%) |
|---|---|---|---|---|---|---|---|---|
| Polyurethane-coated silicone gel implant | Hallock [46] | 2 | N/A | N/A | N/A | N/A | 0 | N/A |
| Injectable calcium hydroxyapatite embedded in a cellulose gel | Evans et al. [47] | 6 | 0.4-1.0 mL | N/A | 6 | N/A | N/A | 100% |
| Hyaluronic acid + labia minora Hyaluronic acid + nipple sharing |
Panettiere et al. [7] | 70 20 |
0.2 mL 0.2 mL |
6.6 6.2 |
12 12 |
5.6 3.5 |
15.2 43.5 |
N/A N/A |
| Artificial bone | Yanaga [48] | 100 | N/A | N/A | N/A | N/A | 19.5 | N/A |
| Polytetrafluoroethylene | Wong et al. [49] | 17 | 3.5 mm | N/A | N/A | 4-5 | N/A | 88 |
| Silicone + C-V flap | Jankau et al. [50] | 30 | N/A | 16.73 | 10 | 11.2 | 33.9 | N/A |
8. ALLOGENIC GRAFT AUGMENTATION
Acellular Dermal Matrix (ADM) not only revolutionized the field of implant-based breast reconstruction but also that of nipple reconstruction [51-55]. Indeed ADMs were shaped differently by various authors and employed as augmentation graft in combination with traditional flap to create sustained projected nipple. ADMs were attractive given their low reabsorption rate with high incorporation rate that helped reduce the risk of infections. ADMs were particularly suited for revisional nipple reconstruction as they can ensure low to mild projection loss at long-term follow-up [3]. Furthermore, given the high cost of ADMs, they can be banked at the time of breast reconstruction in order to cut down related costs [52]. AlloDerm (LifeCell Corporation, Branchburg, NJ, USA) and SurgiMend (TEI Biosciences, Boston, MA, USA) were the ADMs employed by Nahabedian [51], Chen et al. [52], Garramone and Lam [53], Seaman et al. [54], Bramhall et al. [55], and Craft and May [56].
Across all studies, nipple projection loss rate was similar, ranging from 48.8% to 53%; only Craft and May [56] reported loss of nipple projection as low as 25%. Nevertheless, overall complication rate was lower than alloplastic and autologous graft too.
Other allogenic material employed as augmentation grafts for nipple reconstruction were: lyophilized Costal Cartilage and extracellular matrix collagen [57, 58].
Kim and Lee [57] described the use of cadaveric lyophilized costal cartilage along with modified top hat technique. However they observed 57.7% loss of projection at 12 months follow-up, concluding that no added benefit could be achieved when compared with traditional flap only.
Tierney et al. [58] used extracellular matrix collagen of porcine small intestinal submucosa origin (Biodesign Nipple Reconstruction Cylinder; Cook, Inc., Bloomington, IN, USA) shaped into rolled cylinder as augmentation graft along with skate flap for nipple reconstruction. At 6 months, loss of projection ranged from 30 to 50%, and reconstructed nipples were 3 to 5 mm high. Furthermore the only reported complication was cylinder extrusion that occurred in 3.5% of reconstructed nipples requiring revision surgery (Table 5).
| Allograft employed + local flap | Authors | N° Patients | Grafts size | Projection (mm) | Follow-up (months) | Final projection (mm) | Projection loss (%) | Satisfaction rate (%) |
|---|---|---|---|---|---|---|---|---|
| AlloDerm + C-V flap | Nahabedian et al. [51] Chen et al. [52] |
8 11 |
1 x 2 cm N/A |
7-8 NA/A |
6-12 7 |
4-5 N/A |
50% N/A |
N/A N/A |
| AlloDerm + star flap | Garramone & Lam53 | 14 with implant-based reconstruction 16 following TRAM-based reconstruction |
1.5 x 4.5 cm 1.5 x 4.5 cm |
1.2 1.15 |
12 12 |
0.7 0.5 |
53 48.8 |
N/A N/A |
| AlloDerm + skate flap | Seaman et al. [54] | 12 | N/A | N/A | N/A | N/A | N/A | N/A |
| SurgiMend + arrow flap | Bramhall et al. [55] | 13 | 3 x 1 cm + 1 x 1 cm | 10.7 | 12 | 5.2 | 51.4 | N/A |
| SurgiMend + skate flap | Craft & May [56] | N/A | 50 x 50 x 0.2 mm | 8 | 7 | 6 | 25 | N/A |
| Lyophilized costal cartilage + modified top hat flap | Kim & Lee [57] | 17 | N/A | N/A | 12 | N/A | 57.7 | N/A |
| Extracellular matrix collagen + skate flap | Tierney et al. [58] | 83 | 0.7-1 x 1-1.5 cm | 6-7 | 6 | 3-5 | 30-50 | N/A |
9. DISCUSSION
Most NAC reconstruction follow its loss as consequence of breast cancer, but it can be also caused by trauma, congenital absence, burn deformities, and complications from breast surgeries [1]. In case of breast cancer, many surgical techniques and devices have been developed for breast reconstruction to restore an aesthetically pleasing breast shape, however patients may still experience psychological distress until NAC recreation [59-91]. Thus, NAC reconstruction is often the final and completing stage of breast reconstruction for postmastectomy patients [2]. During the last 30 years an enormous amount of techniques has been described given the unsatisfactory results obtained [3]. Indeed, the ideal reconstructed nipple should symmetrically resemble the contralateral one in high and diameter and should match in color and texture. Contralateral color/texture match can be achieved by tattooing, but nipple reconstruction options fail to ensure adequate nipple projection at long-term follow-up [3]. Composite nipple grafts give the best guarantee of color-texture match with the contralateral side and show satisfactory nipple projection even at long-term follow-up [11, 12]. Moreover composite nipple graft is the better choice in case of breast that has undergone implanted-based reconstruction where reconstructed breast skin envelope is usually tight and thin. Indeed, local flaps would be more prone to shrinkage because of tension [11, 12].
Local flaps most commonly used have a loss of projection of up to 70 percent. This is particularly true when reconstruction is under-taken in the thinned dermis seen after tissue expansion or following adjuvant radiotherapy. Nevertheless they give the best outcomes in case of reconstructed breast by means of Latissimus Dorsi flap, followed by gluteal one. Indeed these are the flap that display the thickest dermis layer. Whereas autologous reconstruction with flap raised from the abdominal wall or gracilis flap are more prone to nipple projection loss because of the thinner dermis [2, 3, 30].
Skate, star, C-V, and arrow flap are by far the most commonly employed and the most reliable ones; hence surgeons should be familiar with them and be able to employ the most adequate one on case-to-case basis [10]. Minor morbidity is usually associated, while the main complication is tip necrosis of elevated flaps.
Autologous, allogenic, and alloplastic materials proved to be able to provide sustained projection to reconstructed nipples [12]. Alloplastic grafts were associated with the lowest rates of projection loss followed by autologous and allogenic ones. Nevertheless allogenic grafts are also associated with the highest complication rate, while autologous and allogenic ones have similar rates. Infection, seroma, and fat necrosis are the more commonly reported complications of autologous grafting along with donor site morbidity, while allogenic and alloplastic augmentation grafts may also experience the risk of overcorrection and graft exposure [12].
CONCLUSION
NAC reconstruction is the final and concluding stage of breast reconstruction. Given the numerous techniques described in literature it is clear that the ideal nipple reconstruction hasn’t been found yet. Indeed current techniques are unable to ensure symmetry with the contralateral nipple in high and diameter and color/texture match. However, surgeons should be familiar with most nipple reconstruction techniques since one can be the best choice over another depending on type of mastectomy, radiotherapy, type of reconstruction, skin thickness, tissue condition, and patients’ expectations. Skate, star, arrow, and C-V flap are usually surgeons’ first choice given long-term outcomes. Common traditional flaps can be employed along or in combination of augmentation grafts. Autologous grafts ensure sustained nipple projection but donor site morbidity remains an issue as well as implant extrusion in case of alloplastic grafts. In addition, careful patient evaluation and trustful doctor-patient relationship are essential to achieve satisfying results.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
There is no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


