Factors Predicting Glycemic Control in Type 1 Diabetic Patient
Abstract
Background:
Recent years have been marked by numerous advances in the quality of type 1 diabetes care. However, glycemic control remains suboptimal for many patients with type 1 diabetes. The aim of our study was to identify factors associated with poor glycemic control in type 1 diabetic patients.
Methods:
We studied in a retrospective manner, 188 type 1 diabetic patients, admitted to our department then followed up for at least one year.
Results:
There was a negative correlation between age at diabetes onset and HbA1c value (p=0.02). Adolescents had higher HbA1c value than adults (10.8±2.9% vs. 9.2±2.8%, p=0.02). No relationship was found between number of daily insulin injections and mean HbA1c value. Mean HbA1c was higher in patients with poor compliance to insulin therapy (11.1±3.3% vs. 8.9±2.4%, p<0.0001), in those with less than 3 clinic visits per year (10.7±3.5% vs. 9.0±2.1%, p=0.001), in subjects with lipohypertrophy (10.9±2.5% vs. 9.2±3.4%, p=0.008) and those with known celiac disease (14.5±5.2% vs. 9.6±2.9%, p=0.005).
Conclusion:
Several factors were associated with poor glycemic control in our type 1 diabetic patients. Most of them can be changed in particular by strengthening education strategies.
INTRODUCTION
The Diabetes Control and Complication Trial (DCCT) and the follow-up study Epidemiology of Diabetes Interventions and Complications (EDIC) clearly showed that good glycemic control over a prolonged period delays the onset and slows the progression of microvascular and macrovascular complications in type 1 diabetic patients [1-3]. Recent years have been marked by numerous advances in the quality of type 1 diabetes care including more physiologic insulins, continuous subcutaneous insulin pump therapy, sophisticated blood glucose monitoring and newer education strategies. Nevertheless, glycemic control remains suboptimal for many patients with type 1 diabetes even in developed countries [4-7]. The present study aims to identify factors associated with poor glycemic control in Tunisian type 1 diabetic patients.
MATERIALS AND METHODS
We undertook a retrospective study involving 188 type 1 diabetic patients admitted to the Endocrinology - Diabetology department of the university hospital “La Rabta” between January 1999 and December 2004 and followed up for at least one year. The duration of the study was five years.
Patients with an unclear type of diabetes and pregnant women with type 1 diabetes were excluded.
Data were collected by medical records review regarding:
- Patient’s age
- Patient’s gender
- Diabetes duration
- Diseases associated with diabetes
- Insulin therapy: insulin regimen, number of daily injections, insulin dose, type of insulin
- Insulin therapy adherence: compliance behaviors assessed by the clinician at the time of health care visit. Poor compliance to insulin therapy was defined by intentional reduction in insulin dosage or omission of insulin injections.
- Frequency of clinic visits: number of clinic visits per year
- Practice of self-monitoring
- Presence of lipohypertrophy
- HbA1c at each clinic visit was recorded and used as an index of glycemic control.
HbA1c was measured by high-performance liquid chromatography (HPLC) method.
The study was approved by our hospital ethics committee.
Statistical Analysis was performed using SPSS version 13.0. We assessed HbA1c levels according to patients’ demographic and clinical characteristics using the Student’s t test for continuous variables and χ2 (chi-square) test for categorical data. Pearson's Correlation Coefficient was used to analyze relationships between HbA1c and other quantitative variables. Data were expressed as mean ± standard deviation (SD). Statistical significance was posted at level p<0.05.
RESULTS
Mean age of patients was 28.0±12.2 years (ranges: 1-77 years). They were 102 males and 86 females. One hundred and thirty patients (69.1%) were adults (aged between 20 and 65 years) and 55 (29.2%) were adolescents (aged between 13 and 19 years). The mean age at diagnosis of type 1 diabetes was 25.9±12.5 years (ranges: 1-77 years). One hundred and thirty two patients (70.2%) had newly diagnosed diabetes and 56 (29.8%) had previously diagnosed diabetes with a mean duration of 7.0±6.2 years. The mean duration of follow-up was 3.6±1.5 years (ranges: 1-5 years).
Mean HbA1c value during the overall follow-up period was 9.7±3%. There was a significant negative correlation between age at diabetes onset and mean HbA1c value (p=0.02). Adolescents had significantly higher HbA1c values than adults (10.8±2.9% vs. 9.2±2.8%, p=0.02) (Table 1).
| Follow-up year | Adolescents | Adults | p |
|---|---|---|---|
| First year (n=99) | 9.1±2.3 (n=26) | 7.4±2.1 (n=73) | 0.001 |
| Second year (n=97) | 10.8±3.8 (n=30) | 8.9±2.2 (n=67) | 0.01 |
| Third year (n=82) | 11.4±3.4 (n=24) | 9.6±3.2 (n=58) | 0.02 |
| Fourth year (n=72) | 11.0±3.5 (n=18) | 9.4±2.4 (n=54) | 0.02 |
| Fifth year (n=77) | 10.7±3.2 (n=16) | 9.8±2.8 (n=61) | 0.29 |
In the adolescent’s group, there was numerical but no significant difference in the HbA1c value between boys and girls (10.6±2.8% for boys vs. 11.3±3.1% for girls, p=0.42).
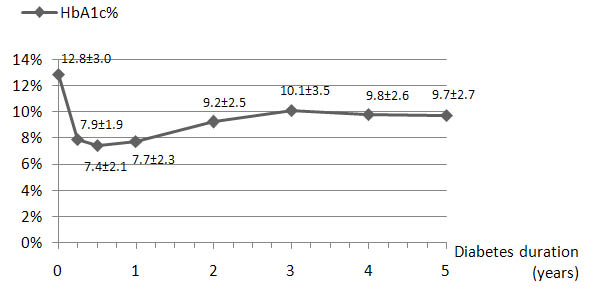
Fig. (1) shows the course of mean HbA1c value during follow-up for patients newly diagnosed with diabetes (n=132). Mean HbA1c was 7.6±2% during the first year of follow-up, and increased significantly (p<0.0001) during the second year to reach a steady-state at 9.2±2.5%.
The insulin treatment regimens initially prescribed included three injections a day in 75.6% of patients, two injections a day in 22.9% of patients and other insulin treatment regimens in 1.6% of patients. The percentage of patients on three daily insulin injections regimens became 58.9% at the end of the five-year study period. Compared to the group treated with twice daily injections regimens, patients on three daily injections regimens were younger and had an earlier onset of type 1 diabetes. No relationship between mean HbA1c value and the number of daily insulin injections was found during the follow-up period (Table 2).
| Three injection insulin regimen (n=142, 75.6%) | Two injection insulin regimen (n=43, 22.9%) | p | |
|---|---|---|---|
| Mean age (years) | 23±7.7 | 39.4±12.1 | <0.0001 |
| Mean age at diabetes onset (years) | 21.7±8.2 | 36.3±13.6 | <0.0001 |
| Mean HbA1c at follow-up (%) | 9.6±2.2 | 9.33±2.2 | 0.53 |
Poor adherence to insulin treatment, a number of clinic visits less than three per year, lipohypertrophy and celiac disease were associated with higher HbA1c levels. Poor adherence to insulin therapy was more frequent in adolescent patients (49.1% of adolescents vs. 33.6% of adults, p=0.04). Patients who practiced self-monitoring (at any frequency) had lower mean HbA1c level than those not practicing it at all. However, the difference was not statistically significant (Table 3).
DISCUSSION
The overall glycemic control of our patients was poor, setting the majority of them at a high risk of diabetes complications. Several factors were significantly associated with this poor glycemic control. Age at clinical onset of type 1 diabetes is one of these factors. In fact, the young age at diagnosis was associated with higher HbA1c during follow-up. This may be explained by the fact that autoimmune β cell destruction is faster and more intensive in patients with early-onset type 1 diabetes mellitus. The process is less aggressive in adults and remnant insulin secretion may facilitate a better metabolic control [8-10].
The period of adolescence is associated with an almost unavoidable deterioration in glycemic control. Our adolescent patients had significantly higher mean HbA1c value compared with adult patients (1.65% higher). This might be explained by an increase in insulin resistance secondary to the hormonal changes of puberty [11]. Furthermore, adolescence is characterized by psychological changes, changes in daily life activities and in eating habits. All this may lead to a poor compliance with treatment [12]. In fact, poor compliance with insulin treatment was more frequent in our adolescent patients.
| Mean HbA1c at follow-up (%) | p | |
|---|---|---|
|
Adherence to insulin treatment Poor (n=74, 39.4%) Good (n=114, 60.6%) |
11.1±3.3 8.9±2.4 |
<0.0001 |
|
Number of clinic visits per year < 3 (n=92, 49%) ≥ 3 (n=96, 51%) |
10.5±3.6 8.9±2.1 |
0.001 |
|
Blood glucose monitoring Practiced (n=20, 10.6%) Not practiced (n=168, 89.4%) |
9.0±2.4 9.7±3.0 |
0.3 |
|
Lipohypertrophy Objectified (n=59, 31.4%) Not objectified (129, 68.6%) |
10.9±2.5 9.2±3.4 |
0.008 |
|
Celiac disease Associated to diabetes (n=3, 1.5%) Not associated to diabetes (n=185, 98.5%) |
14.5±5.2 9.6±2.9 |
0.005 |
Although boys had lower mean HbA1c than girls, the difference was not significant. Numerous studies had shown that girls with type 1 diabetes had a worse metabolic control than boys. Gender differences in metabolic control may be explained by a higher insulin resistance, a higher prevalence of eating disorders and insulin misuse for weight-control purpose in female adolescents with type 1 diabetes [11-13].
Glycemic control is also related to the duration of type 1 diabetes. The lowest HbA1c values were recorded during the first year of diabetes onset and increased significantly over the next years. This is a well-known phenomenon often referred to as the “honeymoon period”. In fact, the residual beta cell function may deliver intrinsic insulin for months to years after diagnosis [12].
With regard to insulin treatment regimen, the Diabetes Control and Complication Trial (DCCT) clearly showed that intensive insulin therapy (three or more injections per day of insulin or continuous subcutaneous insulin infusion) gave better glycemic control than conventional insulin therapy (two injections per day of insulin) [1]. However, in our study we did not find such difference in mean HbA1c between patients treated with twice daily injections regimens and patients treated with three daily injections regimens. This may be explained by tighter follow-up, in the DCCT study, based on self-monitoring of blood glucose and phone call “visits” to better adjust insulin doses.
Our results clearly showed that a poor adherence to insulin treatment was a factor that contributes to poor glycemic control. This finding is consistent with previous studies [11, 14, 15]. Morris et al. demonstrated a direct association between failure to take insulin and poor glycemic control in a cohort of 89 type 1 diabetic patients. There was a significant inverse association between the “adherence index” and HbA1c (p<0.001) [11].
We demonstrated that the mean number of clinic visits per year contributed to a better glycemic control. In fact, the mean HbA1c value was lower in subjects with at least 3 visits per year. Previous reports have shown that type 1 diabetic patients with more frequent clinic visits had better metabolic control [16, 17]. A study of Kaufman et al. showed a significant difference in the mean HbA1c levels between subjects with 1 to 2 visits vs. 3 to 4 visits per year (9.0 ± 2.0% vs. 8.3 ± 1.6%, p<0,05) [16].
Self-monitoring of blood glucose is an essential component of the management of type 1 diabetes. Several studies have shown that a more frequent self-monitoring of blood glucose was associated with lower HbA1c levels [18, 19]. In our study, the mean HbA1c was lower in patients who practiced self-monitoring compared with those not practicing it at all, but the difference was not significant. This is probably due to the small number of patients practicing the self-monitoring. In addition, we have no idea about the quality of this self-monitoring (number of blood glucose determinations, how the patients use the data to adjust food intake, exercise or insulin therapy…).
We have also confirmed findings from other studies reporting that lipohypertrophy was associated with poorer glycemic control [20-22]. In the study of Kordonouri et al., patients with lipohypertrophy had significantly higher HbA1c values (p<0.05) [20]. This complication can be avoided by repeated education of type 1 diabetic patients about adequate insulin injection technique and by encouraging the patient to make regular self examinations. Injection sites should also be examined by health care providers at each clinic visit.
The prevalence of celiac disease in type 1 diabetic patients is higher than in the general population [23-26]. Previous studies, concerning the influence of celiac disease on metabolic control in type 1 diabetic patients are conflicting [23, 26-28]. In our study, patients with type 1 diabetes and a known celiac disease had poorer glycemic control. However, we are limited by the small sample size.
CONCLUSION
Several factors were shown to be associated with poor glycemic control in our type 1 diabetic patients. Although some of these factors are not modifiable, such as age at diabetes onset and diabetes duration; others such as insulin treatment regimen, adherence to insulin therapy, practice of self-monitoring, appearance of lipohypertrophy can be changed and must be targeted by intervention strategies to improve the prognosis of these patients. An intensive patient education remains the cornerstone of a successful treatment.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


