All published articles of this journal are available on ScienceDirect.
CXCR4-containing Exosomes Derived From Cancer Associated Fibroblasts Promote Epithelial Mesenchymal Transition in Ovarian Clear Cell Carcinoma
Abstract
Aims:
Interaction between CAFs and OCCC in the tumor microenvironment and its possible pathway.
Background:
Ovarian clear cell carcinoma (OCCC) has high invasion, metastasis and poor prognosis. Tumor invasion is facilitated by epithelial mesenchymal transition (EMT), which is associated with stromal cells of tumors that mostly consist of cancer associated fibroblasts (CAFs). Exosomes are important carriers of information exchange and transmission between cells.
Objective:
We aimed to investigate whether CAFs can induce OCCC invasion through exosomes.
Methods:
We extracted exosomes with an exosome extraction kit. Immunofluorescence staining was used to detect whether exosomes were internalized by ES2 cells. EMT-related proteins were detected, and invasion experiments were carried out to investigate whether CAFs can induce OCCC invasion through exosomes.
Results:
We found that CAF and NF exosomes could be internalized in ES2 cells. The expression of OCCC CXCR4 protein could be increased by adding the supernatant of CAFs, but there was no significant change in the expression of CXCR4 mRNA. CXCR4 protein expression in CAF exosomes was significantly higher than that in NFs. Enhanced tumor invasiveness in ES2 cells was associated with CAF exosome-mediated, increased levels of N-cadherin and β-Catenin. Inhibition of CXCR4 expression or the Wnt/β-Catenin pathway of ES2 cells potentially reverses EMT and invasion induced by CAF exosomes.
Conclusion:
This study demonstrates that CXCR4-containing exosomes derived from CAFs could promote the EMT and invasion of OCCC.
1. INTRODUCTION
Among epithelial ovarian cancers, ovarian clear cell carcinoma (OCCC) has an incidence rate of 5% [1]. It has the characteristics of non-responsiveness to standard chemotherapy, rapid invasion leading to metastasis and negative prognostic outcomes. However, there are still no effective therapeutic targets. Therefore, we urgently need to find an effective molecular target.
Epithelial mesenchymal transition (EMT) facilitates tumor invasion and metastasis and is correlated with cancer associated fibroblasts (CAFs) which are distinct from normal fibroblasts (NFs) and mainly constitute stromal cells of the tumor microenvironment [2]. CAFs and NFs can be identified and purified by vimentin, keratin and α-SMA [2]. However, the mechanism by which CAF cells promote tumor cell invasion and metastasis is unclear.
Exosomes are double-layer vesicles with a diameter of 30-120nm, which are produced by the fusion of cell multicysts and cell membranes [3, 4]. Exosomes contain protein, fat and nucleic acid, and their bilayer lipid membrane structure can protect these contents from degradation by enzymes, which is an important carrier of information exchange and transmission between cells [5, 6]. Li et al. confirmed the presence of CXCR4, which can trigger an invasion, migration as well as lymph angiogenesis in otherwise benign homologous HCA-P cell pairs in HCA-F cell exosomes of mice with high rates of lymph node metastasis [7]. Therefore, CXCR4 in exosomes may be a new therapeutic target for cancer metastasis.
Cancer with high CXCL12 expression levels can transfer inflammatory cells, blood cells and stromal cells with CXCR4 receptor expression into tumor tissue [8]. Hence the growth of tumors is promoted by cytokine, growth factor, angiogenic mediator and chemokine secretion [9, 10]. Exosomes are considered to be the key messengers of intercellular communication by transferring molecules to receptor cells [11]. In this study, we found that CAFs transport CXCR4 to ES2 cells through exosomes and induce ES2 cell EMT and invasion.
2. MATERIALS AND METHODS
2.1. Reagent Source
The exosome extraction kit was purchased from Shanghai Gefan Biology (Shanghai, China). Wnt/β-Catenin inhibitor XAV939 was purchased from R & D systems (Minneapolis, Minnesota, USA). CXCR4 antibody (11073-2-ap) was provided by Proteintech (Wuhan, Hubei, China). CXCR4 siRNA (sc-35421) was provided by Santa Cruz Biotechnology (Dallas, Texas, USA). Abcam (Cambridge, United Kingdom) supplied all other primary antibodies, including anti-N-cadherin and anti-E-cadherin. Other reagents were provided by Sigma Aldrich (Darmstadt, Germany).
2.2. Cell Culture and Transfection
Clean specimens of OCCC were selected for CAF isolation and culture. After two generations, purified third generation cells were used for preliminary identification. NFs cells were cultured in the same way from normal ovarian clean specimens removed during hysteromyoma operation. The cells were identified by morphological observation and immunocytochemical staining. The separation and purification process has been described in our previous study [12].Written informed consent was provided by the patients with OCCC, and the study was approved by the Research Ethics Committee of Shandong Cancer Hospital and Institute.
ATCC (Virginia, USA) supplied the ovarian cancer cell line ES2. According to the relevant instructions, the cells were propagated 1:2 when they were 70% confluent. The ES-2 cells were cultured in McCoy's5A medium containing 10% fetal bovine serum and 1% streptomycin. CAFs and NFs were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin.
CXCR-4 siRNA (sc-35421) was used to transfect CAFs with Lipofectamine 3000 (Invitrogen, CA). qRT-PCR and WB were used to verify the results.
2.3. Exosome Extraction
Exosomes were extracted using the exosome extraction kit (Shanghai Gefan Biology, Shanghai, China). After successful cell culture, cells were grown in media without exocrine secretions. Cells were centrifuged to isolate supernatants which were in turn centrifuged for 20 min × 3000 g at 4°C. Supernatants were harvested and filtered using a 0.22μm filter. To 1ml of cell culture supernatant, 0.2ml of exosome separation and purification solution were added, then mixed well with a pipette gun. The mixture was placed in the refrigerator overnight at 4°C and subsequently centrifuged at 1500g×30 min at 4°C. After the supernatant was removed, the pellet was centrifuged at 1500g×5min and 4°C to remove residual supernatant.
2.4. Transwell Assay
Cell invasion analyses were performed using a 24-well plate housing a transwell chamber. The top section of the chamber was supplemented with serum-free medium and cell suspension (400μl, 5×104 cells/L), while 10% fetal bovine serum was added to the bottom section in 500μl of culture medium. Cells were cultured under standard conditions for 24 hours, then migrated cells were isolated, fixed with formaldehyde and stained using crystal violet (0.1%). The mean number of migrated cells was estimated by counting cells from 10 random microscopic visual fields.
2.5. Western Blotting
Standard Radio immunoprecipitation Assay (RIPA) extraction methodology was followed to extract total cell proteins using RIPA solution. The bicinchoninic acid assay (BCA) was used to establish the concentration of protein in cell lysates. Membrane blocking to avoid non-specific antibody binding was carried out for 1 hour using 5% defatted milk. This was followed by primary antibody incubation overnight at 4°C. TBS-Tween buffer was used for 3 × 5-minute membrane washes, then stably membrane-bound primary antibodies were probed with secondary antibodies for an hour at room temperature. Chemiluminescence was used to detect antibody signals, which were scanned and detected by a gel imager (Thermo Fisher Scientific).
2.6. Exosome Fluorescence Staining
Exosomes were purified from the fibroblast supernatant. One milliliter of purified exosome suspension and DiO (1 μM) were mixed for 10 min to enable staining. The mixture was sedimented by two cycles of ultra-high speed centrifugation at 100000 g/min at 4°C for 40 min. Labeled exosome pellets were re-suspended with DMEM after removing the supernatant. The labeled exosomes were co-incubated with OCCC ES2 cells for 24 hours. After removing the culture medium, 3 washes with PBS were performed on the cells, followed by ten minutes of paraformaldehyde (4%) fixation and 3 further PBS washes. The nuclei were stained under room temperature conditions for five minutes with DAPI, and the excess stain was removed with 3 PBS washes. ES2 cell internalization of DiO-labelled exosomes was monitored by confocal microscopy.
2.6.1. PCR
The total RNA and miRNA of cells were extracted by Ultrapure RNA Kit (Cwbio,China). cDNA was reverse transcribed using the miRNA cDNA Kit (Cwbio, China) from RNA samples. Then real-time PCR was carried out with the miRNA Real-Time PCR Assay Kit. The expression of CXCR4 mRNA in a given sample is normalized to the small nuclear RNA U6. The relative changes in gene expression were analyzed by GENEWIZ (Beijing, China). The following primers were used: CXCR4 F:5'-TGCAAGGCAGTCC ATGTCAT-3',R:5'-GAAGAAAGCTAGGGCCTCGG-3'. Human U6: F: 5'-CTC GCT TCG GCA GCA CA-3',R:5'-AACGCT TCACGAATTTGCGT-3'.
2.7. Statistical Analysis
We manipulated datasets and performed statistical analyses using version 22 of the Statistical Package for Social Sciences (SPSS; IBM, USA) software. Experimental results are represented as means ± standard deviation. Differences between the two groups were explored using the student’s t test, while one-way ANOVA was used for comparison among larger groups. Statistical significance of results was taken at probability (p) values of P<0.05.
3. RESULTS
3.1. Extraction and Validation of Exosomes
After the exosomes were extracted using the exosome extraction kit, CAF exosomes were found to be half cup-shaped (Fig. 1A) by electron microscopy, and NF exosomes were also half cup-shaped (Fig. 1B). We used a Malvern nanoparticle tracking analyzer to record 10s videos of diluted exosome particle trajectories. Additionally, we used NTA software to analyze particle diameters and concentrations and thereby established that the mean CAF exosome diameter was 176.0nm (Fig. 1C). The mean NFs exosome diameter was 104.8nm (Fig. 1D). There is a slight difference in the average particle size between them, which needs to be further studied. The exosomes were purified from the supernatant of fibroblasts, and the labeled exosomes were incubated with OCCC ES2 cells for 24 hours. The results of confocal microscopy showed that DIO-labeled exosomes were internalized by ES2 cells Fig. (2).

(A) electron microscopy of exosomes in CAFs; (B) electron microscopy of exosomes in NFs; (C) the average size of exosomes in CAFs; (D) the average size of exosomes in NFs.
3.2. CXCR4 in the Supernatant of CAFs was Mainly Located in Exosomes. The CXCR4 Expression in Exosomes Derived from CAF was Significantly More Abundant than that in Exosomes of NFs and CAFs CXCR4 siRNA
CXCR4 siRNA was supplied to CAFs to decrease CXCR4 expression. And the results were confirmed by Western blotting (Fig. 3A). The exosomes of CAFs, NFs and CAFs CXCR4 siRNA were extracted and re-suspended for Western blotting. The result showed that the CXCR4 expression in exosomes derived from CAF was significantly more abundant than that in exosomes of NFs and CAFs CXCR4 siRNA (Fig. 3B).
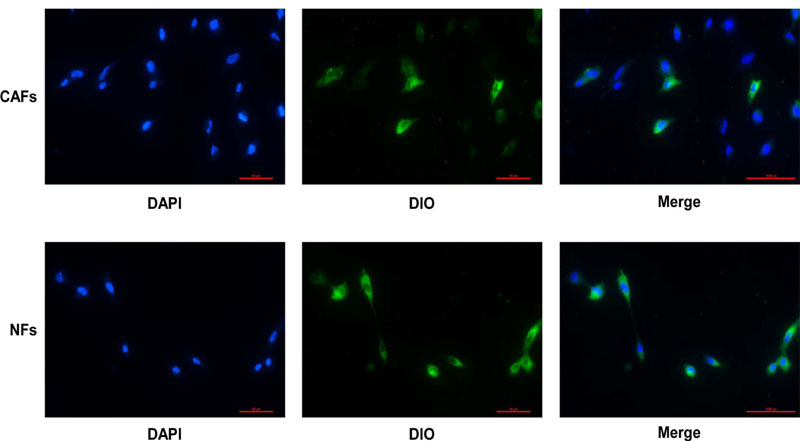
DAPI stained ES2 cells, DOI stained exosomes. The process of CAFs-derived or NFs-derived exosomes entering ES2 cells was detected under a fluorescence microscope.
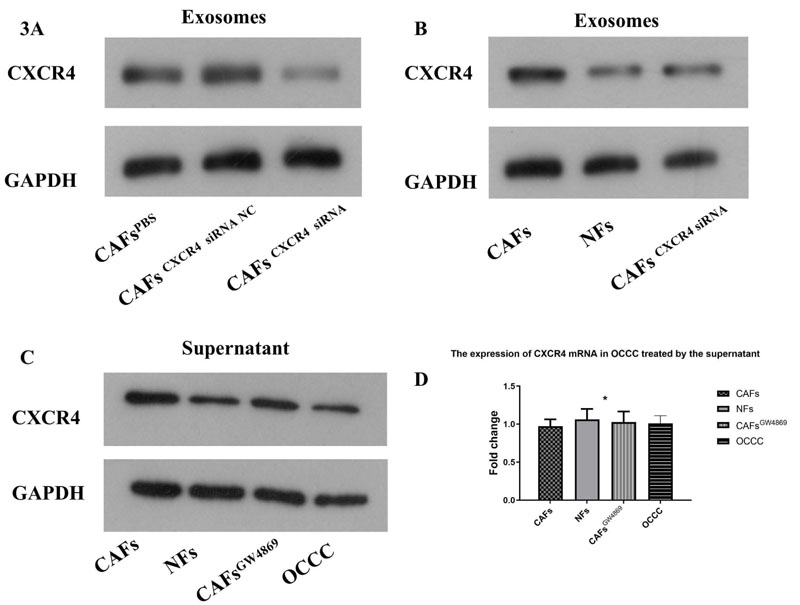
Western blotting confirmed that CXCR4 siRNA could decrease CXCR4 expression in CAFs (A). The exosomes of CAFs, NFs and CAFs CXCR4 siRNA were extracted and re-suspended for Western blotting. The result showed that the CXCR4 expression in exosomes derived from CAF was significantly more abundant than that in exosomes of NFs and CAFs CXCR4 siRNA (B). CAFs cells were treated with exocrine secretion inhibitor GW4869(20 μM, Sigma). The supernatants of CAFs,NFs and GW4869-treated CAFs cells were used to treat OCCC cells, while OCCC cancer cell supernatant was used as a control group. The supernatant derived from CAFs could enhance the expression of CXCR4 in OCCC cells, which could be reversed by inhibiting the secretion of CAFs-derived exosomes with GW4689, while the supernatant derived from NFs could not show the corresponding trend (C). It was found that the expression of OCCC CXCR4 protein increased by adding the supernatant of CAFs, but there was no significant change in the expression of CXCR4 mRNA in OCCC (D).
CAFs cells were treated with exocrine secretion inhibitor GW4869(20 μM, Sigma). The supernatants of CAFs,NFs and GW4869-treated CAFs cells were used to treat OCCC cells, while OCCC cancer cell supernatant was used as a control group. The supernatant derived from CAFs could enhance the expression of CXCR4 in OCCC cells, which could be reversed by inhibiting the secretion of CAFs-derived exosomes with GW4689, while the supernatant derived from NFs could not show the corresponding trend (Fig. 3C). It was found that the expression of OCCC CXCR4 protein increased by adding the supernatant of CAFs, but there was no significant change in the expression of CXCR4 mRNA in OCCC (Fig. 3D).
3.3. Exosomes from CAFs Induce EMT and Invasion of ES2 Cells by Delivering CXCR4
In addition, CAF supernatant with exosome removal (CAFs supernatant group) was assigned as a control group. Transwell assay results showed that compared with exosomes from NF and CAFCXCR4 siRNA groups, exosomes from CAFs significantly enhanced invasiveness in ES2 cells (Fig. 4A). Western blotting results showed that compared with ES2 cells treated by the NF exosome group or CAFCXCR4 siRNA exosome group, the expression of EMT transcription factor β-catenin and interstitial protein N-cadherin protein increased significantly in ES2 cells treated by CAFs exosome (Fig. 4B). This suggests that CAFs may promote the invasion of OCCC through EMT, which needs to be verified by further experiments.
3.4. Blocking EMT with Wnt/β-Catenin Pathway Inhibitor XAV939 could Inhibit the Invasion Induced by CAFs Exosomes
The exosomes from CAF cells and CAF CXCR4 siRNA cells were added to ES2 cells, respectively. Then, the proteins related to EMT, N-cadherin and E-cadherin were detected. Western blotting demonstrated that N-cadherin expression significantly increased in ES2 cells treated with CAF exosomes compared to that treated with CAFCXCR4 siRNA exosomes, while E-cadherin expression illustrated the opposite trend, suggesting that exosomes containing CRCX4 contributed to EMT. Wnt/ β-Catenin pathway is one of the classical pathways related to EMT. When we treated ES2 cells using both exosomes from CAF cells and Wnt/β-Catenin inhibitor XAV939 (10 μM; Selleck, USA), western blotting showed that EMT, induced by CAF exosomes, could be inhibited by XAV939 (Fig. 5A). Finally, transwell assay confirmed that CAF exosomes containing CRCX4 could promote the invasion of ES2 cells (Fig. 5B). When EMT was reversed by XAV939, the invasive ability of OCCC cancer cells was inhibited.
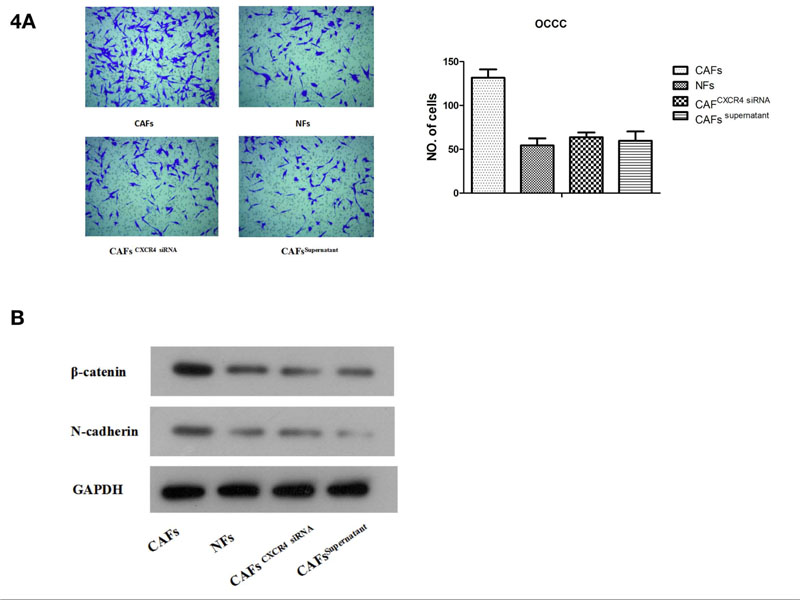
(A) A Transwell assay was used to measure the invasive capacity of ES2 cells after adding CAF exosomes or CAF CXCR4 siRNA exosomes.(B) Western blotting was used to detect the protein expression of β-catenin and N-cadherin after adding CAF exosomes or CAFCXCR4 siRNA exosomes.
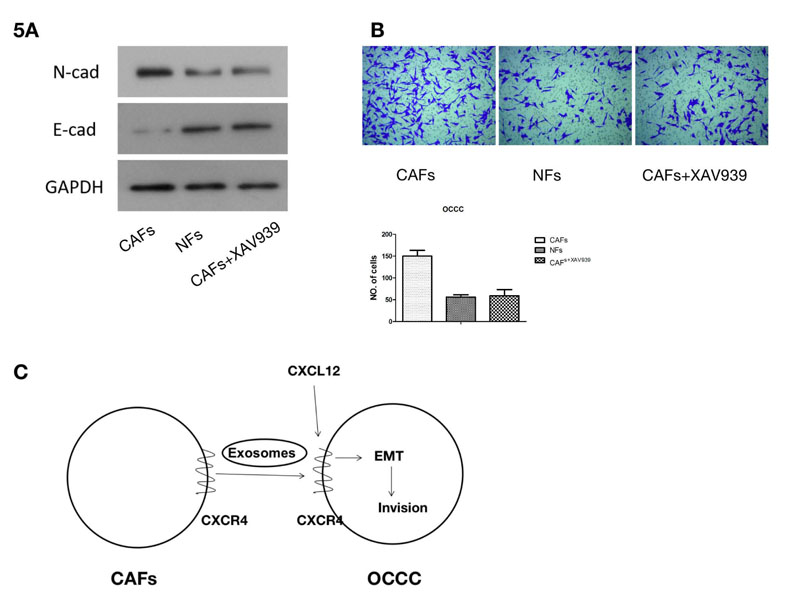
(A) Western blotting was used to detect the protein expression of β-Catenin and N-cadherin after the Wnt / β-Catenin pathway inhibitor XAV939 was added. (B) A Transwell assay was used to detect the change in the invasion ability of ES2 cells following the addition of the Wnt/β-Catenin pathway inhibitor XAV939. (C) Pattern diagram. CXCR4-containing exosomes derived from cancer associated fibroblasts promote epithelial mesenchymal transition in ovarian clear cell carcinoma.
4. DISCUSSION
In addition to mediating material exchange, signal transduction and information exchange between cells, exosomes critically contribute to various physiological and pathological processes of the human body [13]. Tumor-derived exosomes are important regulators of tumorigenesis, invasion, metastasis, and immune escape [14]. Tumor inflammatory microenvironment is closely related to the occurrence and development of cancer, which is more significant in patients with HIV [15]. In the era of precision medicine that advocates personalized diagnosis and treatment, research and analysis of tumor exosomes critically facilitate early detection, therapy and tumor prognostication. CXCR4 is a specific receptor of CXCL12, which mediates several pathophysiological activities, including angiogenesis, inflammation and tissue immunomonitoring [16]. Our results revealed that CAFs can transfer exosomes to ES2 cells through immunofluorescence experiments. Consistently, we found that CAF exosomal CXCR4 was significantly higher than that of NFs. The supernatant derived from CAFs could enhance the expression of CXCR4 in OCCC cells, which could be reversed by inhibiting the secretion of CAFs-derived exosomes by GW4689, while the supernatant derived from NFs could not show the corresponding trend. It is suggested that the supernatant derived from CAFs can enhance the expression of CXCR4 in OCCC cells through exosomes. However, there was no significant increase in CXCR4 miRNA in OCCC cells, suggesting that CAFs-derived exosomes may directly transfer CXCR4 to OCCC cells, rather than inducing OCCC cancer cells to over-express CXCR4.
CAF-derived exosomes can induce proliferation, EMT, and invasion in various cancers, which may be potential targets for improving the efficacy of tumor treatment [7, 14, 17]. Guo et al. demonstrated cisplatin resistance could be attributed to CAF-derived exosomal over-expressing miR-98-5p through down-regulation of CDKN1A protein in ovarian cancer [17]. Western blotting and Transwell experiments revealed that after CAFs-derived exosomes were removed, the invasiveness of ES2 cells decreased. Therefore, we established an enhanced invasive ability of ES2 cells transfected with CAF-extracted exosomes.
CXCL12 / CXCR4 signal transduction stimulates several biological mechanisms, including angiogenesis, tumor growth and metastasis, tumor resistance and invasive migration [18] [19]. OSCC [oral squamous cell carcinoma)-extracted CAF exosomes in malignant tissues were reported to highly express CXCR4 by Li X et al. [20]. In addition, CXCL12 expression is elevated in ES2 cells. In previous experiments, we confirmed that CXCR4 was highly expressed in CAFs-derived exosomes and may be transmitted to OCCC cells through exosomes. Next, we questioned whether CAFs could induce EMT and invasion of ES2 cells by transmitting CXCR4. Through Western blotting and invasion experiments, we found that following the addition of CAF-derived exosomes, the expression of CXCR4 protein, the EMT transcription factor β-catenin, the interstitial protein N-cadherin and cell invasion capacity all increased simultaneously. Therefore, we found that CXCR4 transmitted by exosomes is responsible for inducing the EMT and invasion of ES2 cells.
EMT, together with both cancer and embryonic development, vitally depends on Wnt-mediated signaling. It enhances ovarian cancer growth along with EMT and invasion [21, 22]. Similarly, by blocking the Wnt/β-Catenin pathway, we showed diminished EMT and invasiveness induction in ES2 cells via exosomal CXCR4. Therefore, CAF-extracted exosomal CXCR4 may mediate the invasion of OCCC through EMT.
CONCLUSION
In summary, we found that CAF-derived exosomal CXCR4 can induce OCCC EMT and metastasis (Fig. 5C), Pattern diagram). In the future, we will extend our investigations through experiments in vivo and other studies with an aim to unveil new mechanisms for targeted tumor therapy.
LIST OF ABBREVIATIONS
| OCCC | = Ovarian Clear Cell Carcinoma |
| EMT | = Epithelial Mesenchymal Transition |
| CAFs | = Cancer Associated Fibroblasts |
| RIPA | = Radio Immunoprecipitation Assay |
| BCA | = Bicinchoninic Acid Assay |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical approval and clearance were received from the Ethical Committee of Shandong Provincial Hospital, China (SWYX:NO. 2021-318).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Written informed consent was provided by the patients with OCCC.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This study was financially supported by the Natural Science Foundation of Shandong Province (Grant No. ZR2019PH111 and ZR2020LZL012) Key Research Program of Shandong Province (Grant No. 2020RKC03013), Clinical Medical Science and Technology Innovation Plan of Jinan Science and Technology Bureau(Grant No. 202019001), Clinical Research and cultivation Project of Shandong Cancer Hospital (Grant No. 2020024), CSCO-CSPC Cancer Research Fund Project (Grant No.Y-SY2021QN-0152), Beijing Science and Technology Innovation Medical Development Foundation (Grant no. KC2021-JX-0186-138) and Clinical Research Fund of Shandong Medical Association (Grant no. YXH2022ZX02149).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


