All published articles of this journal are available on ScienceDirect.
Fibrin-Specific Thrombolytic Therapy for Acute CVA within 6 Hours of Onset, Systematic Review and Meta-analysis
Abstract
Objective:
Cerebrovascular Accident (CVA) remains a major cause of disability and death and fibrinolytic agents might reduce long-term disability. We sought to determine whether patients receiving fibrin-specific thrombolytic agents acutely (within 6 h) following CVA had improved functional outcome, or decreased mortality or increased intracerebral bleeding at 6-months than patients receiving placebo.
Materials and Methods:
We conducted a systematic review of randomized controlled clinical trials that assessed 6-months functional outcome, mortality and intracranial hemorrhage and compared thrombolytic therapy with placebo in patients randomized within the first 6 hours following CVA. We searched these databases: MEDLINE (1990-2018), Cochrane Central Register of Controlled Trials, and Cochrane Database for Systematic Reviews. Two blinded reviewers reviewed the eligible articles and rated study quality using the Jadad score. We calculated pooled Odds Ratios (ORs) using a random effect model.
Results:
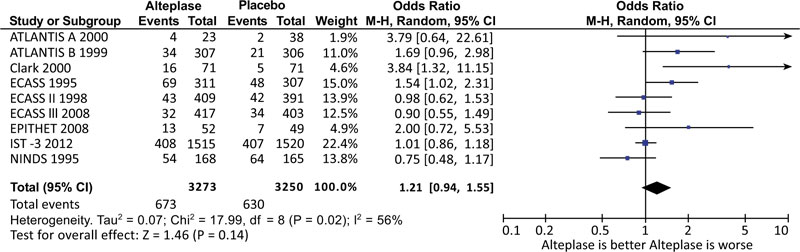
We included 9 studies with 6523 enrolled participants and had 673 deaths. Compared with placebo, thrombolytic therapy within 6 hours of CVA did not result in a statistically significant reduction in 6-month mortality (OR 1.21, 95% confidence interval [CI] 0.94–1.55). More patients in the thrombolytic therapy group had favorable functional outcome (OR 1.20 confidence interval [CI] 1.07–1.35). Thrombolytic therapy caused more fatal intracerebral bleeding than placebo (OR 5.61 confidence interval [CI] 3.40-9.24).
Conclusion:
Fibrin-Specific thrombolytic within 6 hours of CVA improves functional outcome at the expense of increasing symptomatic and fatal intracerebral bleed. Future studies are required before extending the thrombolytic window to 6-hours.
1. INTRODUCTION
Stroke remains a leading cause of death and disability worldwide. Every year, 610,000 new people develop stroke in the United States of America and 140,000 die, making it the fifth leading cause of death [1]. Acute ischemic stroke accounts for 87% of all strokes and hence, restoring blood circulation to the ischemic area of the brain remains a very appealing treatment modality. There has been increasing use of thrombolytic agents for the treatment of acute ischemic stroke [2]. The NINDS study group conducted the first trial that established the use of intravenous recombinant tissue Plasminogen Activator (rt-PA) (Alteplase) as a thrombolytic agent for acute ischemic stroke [3]. Multiple studies have been conducted after this trial to investigate thrombolytic therapy for acute ischemic stroke. Most of these trials showed a trend towards an improvement in the functional outcome at the expense of increasing symptomatic intracranial hemorrhages. The results of various trials encouraged various stroke scientific bodies to approve rt-PA as a standard of care within the first three hours of symptoms onset.
The American Stroke Association expanded the recommendation to 4.5 hours of stroke onset after discussion of the risks and benefits with the patients [4]. Mechanical thrombectomy for acute ischemic stroke has commenced a new era of stroke management for a specific group of patients in the capable centers. A trial including patients who were last known to be well 6-24 hours earlier and had a mismatch between clinical deficit and infarct compared mechanical thrombectomy plus standard of care to the standard of care alone found better functional outcome in the mechanical thrombectomy group [5]. Based on this study, mechanical thrombectomy is recommended for patients with acute ischemic stroke due to a large artery occlusion who can be treated within 24 hours of symptoms onset. Patients are still eligible for rt-PA within the thrombolytic window, even if thrombectomy is being considered [6].
The previous systematic review investigated the safety and efficacy of thrombolytic agents when administered within 4.5 hours [7], which showed improvement in the functional outcome. Another review, which included studies investigating various thrombolytic agents, concluded that there is evidence of increased early mortality and rates of symptomatic intracranial hemorrhage with improved functional outcomes [8]. More research was thought to be needed to further assess the functional outcome and the mortality benefit of thrombolysis in acute stroke.
Given these recent developments in acute ischemic stroke care and questionable safety of delayed thrombolysis among studies, we conducted this systematic review to assess the functional outcome and mortality benefit in patients treated with fibrin specific thrombolytic agents for acute ischemic stroke within 6 hours of symptoms onset.
2. METHODS
2.1. Search Strategy
We searched MEDLINE (1990–2018), and Cochrane Central Register of Controlled Trials. We used the RCT filter and limited our search to English language articles. We searched for fibrin specific thrombolytic therapy and acute stroke. We used synonyms for acute stroke, as cerebrovascular accident(CVA), ischemic stroke, and stroke. We searched for the specific thrombolytic agents, including alteplase(t-PA), tenecteplase(TnK), reteplase (RPA), and desmoteplase. We used Boolean Operators “AND, OR” to connect the different search terms. We used the operator “OR” to connect the various thrombolytic agents. We also combined the CVA synonyms using “OR” operator. Then, we used the operator “AND” to combine the thrombolytic agents and CVA synonyms.
2.2. Inclusion and Exclusion Criteria
We included RCTs that randomized acute CVA patients within 6 hours of symptom onset to either intravenous fibrin-specific thrombolytic therapy or a control group. The control group received either a placebo or no additional treatment over routine care. Studies must have included outcome measures within the first 6 months of enrollment. We excluded studies with intra-arterial thrombolytic therapy or other non-fibrin specific thrombolytic therapy.
2.3. The Outcome Measures
We assessed the following outcome measures in the review: death, disability and symptomatic or fatal intracerebral bleed. The outcome could be a primary outcome or secondary outcome in the original trials. The disability is assessed using the modified Rankin Scale(mRS) or Oxford Handicap Scale(OHS). Both scales are quite similar. A scale of three or more (3+) constitutes a significant disability with a higher number indicating more disability.
2.4. Study Selection
We reviewed all citations and screened the citations using the inclusion and exclusion criteria. Two reviewers (M.R, A.M) reviewed the abstracts and selected the articles for the full review. Disagreements regarding study selection were resolved by consensus.
2.5. Data Extraction
We abstracted the data from the selected articles into a Microsoft excel. Abstracted variables included study characteristics such as study population, inclusion and exclusion criteria, intervention, outcomes, and entry period into the study. Two reviewers(F.A, H.R) reviewed all the included articles and abstracted the outcome and the study characteristics.
2.6. Assessment of Methodological Quality
Two reviewers (M.R. and A.M) independently assessed and summarized the quality of each trial using the Jadad score. They rated each article based on the score from 1-5. The score is used to assess the methodological quality of trials considering randomization, blinding and the dropouts of each article. Discrepancies were resolved by consensus with a third reviewer available if needed.
2.7. Statistical Analysis
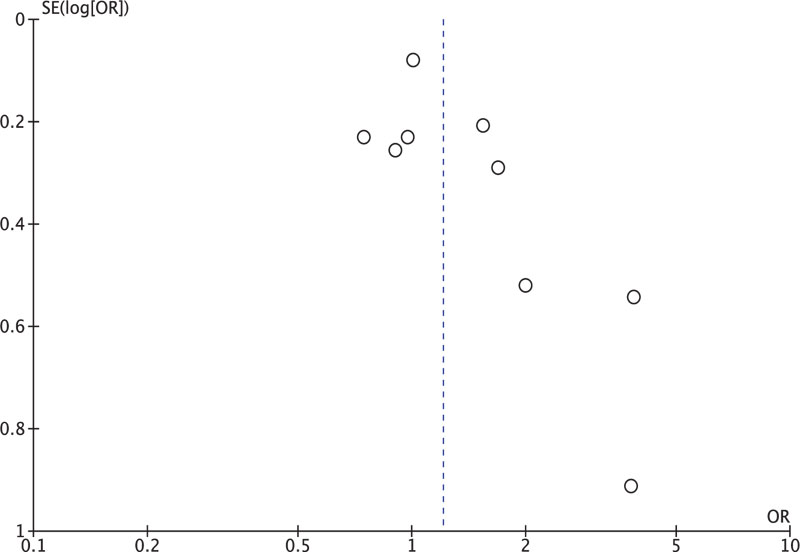
We calculated the odds ratio (OR) for the primary outcome using RevMan Analyses statistical software version 5.3. (The Cochrane Collaboration, Oxford, England). A p-value of < 0.05 was considered statistically significant. We calculated the OR for disability, mortality, symptomatic and fatal intracerebral bleeding. We used a random-effects model to account for the variability among the trials. We assessed heterogeneity by examining study characteristics such as population, settings, intervention given, length of follow-up and outcome assessment. We used I2 statistic to quantify statistical heterogeneity across studies. An I2 value greater than 50% indicates substantial heterogeneity. A funnel plot was constructed to assess for evidence of publication bias.
3. RESULTS
3.1. Study Selection
We searched Medline and Cochrane Central Register of Controlled Trials. We identified 1280 original citations using our search strategy. We retrieved 14 full articles and then excluded 5 articles. Nine studies met our inclusion criteria and included in the final results, as shown in Fig. (1). The reviewers agreed on all the study selection.
3.2. Review Description
We included 9 RCTs in our review. The study characteristics are summarized in Table 1. The first study was published in 1995(NINDS) and the latest was in 2012(IST-3). The sample sizes ranged from 61 to 3035 patients (a total of 6523 patients). All studies were RCTs. All studies followed similar inclusion and exclusion criteria. All studies included patients with definite symptoms and signs of stroke within a clear time onset, had no intracranial bleeding on non-contrast CT scan or MRI. All trials excluded patients with a high risk of bleeding. All trials reported mortality, disability (all reported mRS except IST-3 reported OHS=Oxford Handicap Scale), and symptomatic and fatal ICH.
3.3. Outcome Synthesis
A total of 673 patients died in the intervention group compared to 630 patients among the placebo group. However, thrombolytic therapy within 6 hours after CVA did not result in a statistically significant reduction in 6-month mortality (OR 1.21, 95% [CI] 0.94–1.55) (Fig. 3). More patients in the thrombolytic therapy group had favorable functional outcome (OR 1.20 [CI] 1.07–1.35) (Fig. 2). This means having a less disability as measured by the mRS or OHS. Thrombolytic therapy caused more symptomatic intracerebral bleeding (OR 5.36 [CI] 3.78-7.62). and more fatal intracerebral bleeding than placebo (OR 5.61 [CI] 3.40-9.24) (Fig. 4).
| Intervention | CVA* Symptoms Onset | Sample Size | JADAD Score | |
|---|---|---|---|---|
| NINDS 1995 [3] | Alteplase (tPA) | 3 hrs | 624 | 4 |
| IST-3 2012 [12] | Alteplase (tPA) | 4.5 hrs | 3035 | 3 |
| EPITHET 2008 [13] | Alteplase (tPA) | 3-6 hrs | 101 | 4 |
| ECASS III 2008 [14] | Alteplase (tPA) | 3-4.5 hrs | 821 | 4 |
| ECASS II 1998 [15] | Alteplase (tPA) | 6 hrs | 800 | 5 |
| ECASS 1995 [16] | Alteplase (tPA) | 6 hrs | 620 | 4 |
| Clark 2000 [17] | Alteplase (tPA) | 6 hrs | 142 | 4 |
| ATLANTIS B 1999 [18] | Alteplase (tPA) | 3-5 hrs | 613 | 5 |
| ATLANTIS A 2000 [19] | Alteplase (tPA) | 3 hrs | 61 | 4 |

RCT=Randomized Controlled Trial.

CI=Confidence Interval.
C.I=Confidence Interval.
4. DISCUSSION
Our systematic review included a total of 9 studies and 6817 patients. All the trials included in the review satisfied high-quality scores using the JADAD scale. We used the random effect model as a more conservative approach of estimating the outcome of interest and adjusting for the variation between trials. The metanalysis provides good evidence that Alteplase given within 6 hours of acute ischemic stroke improves functional outcome at the expense of increased intracranial hemorrhage (fatal and symptomatic). Contrarily, the review does not support survival benefit at 6-months post thrombolytic therapy.
Our review demonstrated benefit beyond the current recommendations of 4.5 hours. The overall (OR for the functional outcome is 1.20 confidence interval [CI] 1.07–1.35) favoring intervention. The patients receiving thrombolytic therapy are likely to have less disability as measured by mRS or OHS. The I2 score of 42% reflects a moderate statistical level of heterogeneity. Most of the studies have similar inclusion and exclusion criteria and hence have less clinical heterogeneity. Thrombolytic therapy within 6 hours post-stroke onset, resulted in a relative risk improvement RRI of 4%. Previous reviews demonstrated a diminishing benefit of Alteplase on the functional outcome with delayed administration. One review showed an RRI of 16% in the group of 3-4.5 hours and 6% for the group of delay longer than 4.5hours [7]. Although they found similar results and included most of the same trials, we simplified the question by searching at outcomes for patients within 6-hours of symptoms onset. In addition to Alteplase, we also searched for other fibrin specific thrombolytic therapy. Our search failed to find trials, which included other thrombolytic therapy that meets our criteria. Another review, which had a wider inclusion criterion, indicated a benefit with rtPA given within 6 hrs of ischemic stroke in the form of being alive and independent [8]. In contrast to our review, which replicates the usual clinical practice, they included some trials which used angiography to diagnose and verify the outcome of treatment in patients with CVA.
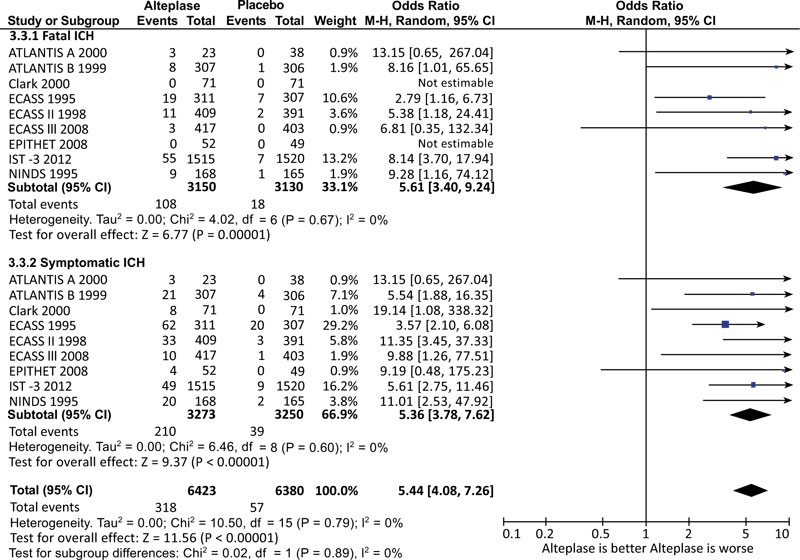
This metaanalysis demonstrated the known increased risk of fatal and symptomatic intracerebral hemorrhage (ICH) with OR of 5.61 and 5.36, respectively. For both analyses, the I2 score was 0%, demonstrating a minimal statistical heterogeneity. One review reported the rate of ICH had not been affected by the delay in giving Alteplase and revealed the risk of ICH is highest in the group with Alteplase administered within 3 hours [7]. Despite the increased risk of ICH, we did not find a statistically significant increase in mortality for patients who received thrombolytic therapy, which is similar to the results reported by the previously mentioned review.
5. LIMITATIONS
Our review has various limitations. The Funnel plot (Fig. 5) for mortality reflects a gap in the area of smaller studies, which might indicate small negative studies are likely not to get published. Despite being a limitation, we expect that if any studies were supporting such a benefit to be published. Our review is limited by the search only in English literature and predominantly on PubMed and Cochrane libraries. It may be missing some studies in other languages or on other libraries; however, we expected if they were any large RCT’s to have been referred to in our reference review. Two studies were found by a detailed reference review but failed to fulfill our criteria assessment [9, 10]. Another limitation is that the outcomes studied are short-termed (within six months only) with no long-term follow up. We expect all important outcomes for such treatment would have been reported within the study period.
We used the random effect model in our meta-analysis. It is a more conservative approach to consider variability between different trials. We did an exploratory analysis using a fixed-effect model, which did not significantly alter our inference results. A trial challenged the time limit for endovascular thrombectomy extending it beyond the 6 hours based on penumbral mismatch on MRI [5]. It revealed a benefit of functional independence at 90 days with no change in the symptomatic ICH rates. Another trial looked at extending the time limit for thrombolysis beyond the current 4.5 hours up to 9 hours based on identifying penumbral mismatch on MRIs to improve functional outcomes for late presenting stroke patients [11-19]. Results have not been published at the time of writing this manuscript.
CONCLUSION
Our review provides a good direction towards the possibility of extending the time window for administering Alteplase up to 6 hours with similar risks of ICH, perhaps with even better results utilizing MRI perfusion to better identify the right patients who would benefit from such therapy.
AUTHORS' CONTRIBUTION
Dr. Abdullah Al Reesi contributed by writing the proposal, specifically the method section, performed the analysis and writing of the results and reviewed the manuscript.
Dr. Amal Al Mandhari and Dr. Marwan Al Raisi Reviewed the abstracts to be included in the review and summarized them. They contributed to the quality rating of the included studies and writing the manuscript.
Dr. Mahmood Al Jufaili, Fahad AL Abri and Huda AL Ruqaishi contributed to reviewing all the included trials, applying the inclusion and exclusion criteria and abstracting the data. The reviewed the manuscript.
All authors were involved in reviewing the manuscript and editing before the final submission. They gave permission to submit the manuscript for publication.
CONFERENCE PRESENTATION
A poster presentation at the International Conference of Emergency Medicine 2019 (ICEM) in Seoul, Korea.
CONSENT FOR PUBLICATION
Not applicable.
STANDARD FOR REPORTING
PRISMA guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


