All published articles of this journal are available on ScienceDirect.
A Primary Care-based Collaborative Hepatitis C Clinic: Clinical Structure and Virologic Outcomes with Direct Acting Antiviral Therapy
Abstract
Background:
Currently 4 million persons in the US have active hepatitis C virus (HCV) infection and most have never successfully completed antiviral treatment. Newer therapies herald potential for wider uptake and acceptance of treatment, but the number of hepatology specialists is limited and newer models are needed to increase access to care. The aim of this study is to describe a collaborative primary care-based clinic for HCV treatment.
Methods:
Retrospective analysis of a collaborative primary care clinic developed for the evaluation and treatment of patients with chronic hepatitis C at one VA medical center. A half-day clinic was organized with 4 primary care MDs, 2 hepatologists, 2 nurse practitioners, and a co-located psychiatrist, pharmacist and nurse case manager. Clinic productivity and outcomes related to the number of patients who initiated and completed treatment with direct acting antivirals (DAA) and pegylated interferon and ribavirin were evaluated.
Results:
In this 18 month period, the clinic had 1890 confirmed HCV registry patients and 1690 clinic visits. 74 HCV genotype 1 patients initiated DAA therapy. Primary care providers treated 47 patients (32% cirrhotic) and hepatologists treated 27 patients (48% cirrhotic). Final SVR rate was 54.6% (39.2% cirrhotics vs. 65.2% noncirrhotics). SVR rates were higher in patients with primary care providers (61.7%) vs. hepatologists (44.4%). Despite numerous adverse events, early treatment termination for adverse events occurred in 5.3% vs. 21.3% for virologic non-response. Multivariate analysis revealed no significant differences between primary care and hepatology for SVR and treatment discontinuations.
Conclusion:
This clinic demonstrated effectiveness and safety with DAA therapy. This illustrates potential for a primary care based collaborative clinic, which will be crucial for expanding access to effective HCV care.
INTRODUCTION
Currently 4 million persons in the US have active hepatitis C infection and world-wide there may be as many as 170 million people with this infection [1]. Patients in the Department of Veterans Affairs (VA) have a high prevalence of HCV infection, approximately 5.4%, which is about 3 times greater than the percentage in the general U.S. population [2]. The importance of antiviral therapy has been emphasized by numerous reports of significant long-term reductions in liver-related mortality following successful antiviral therapy [3-5]. The advent of new direct acting antiviral (DAA) treatment in 2011 represented a breakthrough in increased sustained virologic response rates over the standard therapy of pegylated interferon and ribavirin [6, 7]. Emerging DAA treatments that do not include interferon and have SVR rates approaching 90% or greater herald the potential for wider uptake and acceptance of treatment [8-10]. Due to the numbers of patients with chronic hepatitis C that require antiviral treatment and the potential for significantly greater tolerability of treatment, new models for efficient delivery of care for hepatitis C patients are needed [11]. Primary care providers represent an important resource to expand access to hepatitis C care, but few models of collaborative primary care hepatitis C practices exist. The purpose of this study is to describe a primary care based collaborative clinic for the treatment of patients with hepatitis C, and to describe the initial results achieved by this care model in the use of new DAA treatments for hepatitis C.
METHODS
This was a retrospective analysis of a collaborative primary care clinic designed for the evaluation and treatment of patients with chronic hepatitis C at a single VA medical center. Electronic medical records were reviewed with a uniform data collection tool. Clinical outcomes related to the number of patients who initiated and completed treatment with DAA and pegylated interferon and ribavirin were reviewed from July 2011 through December 2012. The study did not include patients seen in the clinic that were not started on antiviral therapy during this period. The study was approved by the local Institutional Review Board and Research and Development committees.
A single half-day clinic was organized with 4 primary care MDs, 2 hepatologists, 2 nurse practitioners and a nurse case manager. Permission for primary care involvement was negotiated with the Primary Care service line administrators, due to the large number of HCV patients in the system and the need for increased access to care. Volunteers among primary care providers were recruited to participate. The clinic was organized along an integrated care model as previously described [12]. A co-located psychiatrist and pharmacist were integrated into the clinic, and bi-monthly noon meetings were held to discuss treatment issues. Patients were considered candidates for treatment based on published AASLD and VA hepatitis C treatment guidelines. Primary care providers were familiarized with current HCV assessment and treatment guidelines. Decisions to start and adjust antiviral therapy were considered collaboratively with the clinic hepatologists. Patients with psychiatric and/or substance use co-morbidities were co-managed in consultation with the clinic psychiatrist, using model of co-managed care [12]. Colocalization of clinic personnel facilitated communication and patient “hand-offs”, with same-day psychiatry appointments available as needed for patients undergoing treatment.
Patients were screened for substance abuse, psychiatric symptoms, and medical co-morbidity at their initial assessment. The degree of medical co-morbidity was summarized using the Charlson Comorbidity Index [13, 14]. Those patients with a history of psychiatric disease, history of substance abuse disorder (SUD), or current substance abuse were further evaluated by the clinical psychiatrist, prior to initiating anti-virals. Patients with current substance abuse were provided brief intervention and follow-up sessions, with referral to the institution’s drug treatment program when indicated, in order to treat and optimize the patients for subsequent anti-viral therapy. Patients starting anti-viral treatment were also screened for psychiatric symptoms utilizing the Beck Depression Inventory (BDI) prior to, and then periodically throughout, the course of treatment [15]. BDI scores above pre-established thresholds or with significant change triggered individual assessment and treatment by the clinic psychiatrist. Facilitated evaluations were available for patients experiencing acute or emerging psychiatric symptoms while on antiviral treatment. Clinic providers used published VA hepatitis C treatment guidelines for evaluating, initiating, and adjusting antiviral treatment [16]. These guidelines included the requirement that SUD and psychiatric disorders were stable and that patients were able to demonstrate compliance with appointments and recommendations.
Patients were assessed at baseline and generally every 2-4 weeks during antiviral treatment for treatment durations of up to 48 weeks. Clinical assessments were performed by the clinic primary care providers, nurse practitioners, or hepatologist. Patients experiencing adverse effects of therapy, or exacerbation of co-morbid psychiatric or medical disease, received appropriate treatment through the HCV clinic throughout the course of treatment. Sustained virologic response (SVR) was assessed at 12 weeks after completing anti-viral treatment.
Descriptive statistics were used to summarize baseline characteristics. Univariate and multivariate analyses were used to assess the primary and secondary outcomes. Multivariate logistic regression was used to assess the difference in SVR between the two groups with adjustment for baseline characteristics. All analyses were performed by SPSS and R and pvalue<0.05 is interpreted as statistical significant.
RESULTS
This clinic had 1890 confirmed HCV registry patients at the time of the study. During the 18 month time period of the study there were a total of 1690 clinic visits. Clinic capacity included 215 patient slots per month. Same week appointment access was provided. During this time 74 patients with HCV genotype 1 initiated DAA antiviral therapy, 47 patients with Primary Care providers and 27 patients with Hepatology providers. The patients in this cohort demonstrated a high level of co-morbid disease, as demonstrated by the high prevalence of elevated Charlson scores ≥ 3 (Table 1). Psychiatric and substance use disorder co-morbidities were very prevalent in this patient population (Table 2). In the Primary Care provider cohort 75% of patients had psychiatric diagnoses and 75% had SUD diagnoses, the majority of the latter consisted of multiple different SUD diagnoses Table 1. In the Hepatology provider cohort 55% of patients had psychiatric diagnoses and 62% had SUD diagnoses. Only 10.6% in the Primary Care cohort and 18.5% in the Hepatology cohort had no psychiatric or SUD diagnoses listed.
Patient characteristics according to treatment group.
| Patient Characteristics | Total patients (n=74) | Primary Care (n=47) | Hepatology (n=27) | p value |
|---|---|---|---|---|
| Age | 57.1 | 58.0 | 0.609 | |
| Race 1. White, Non Hispanic 2. White, Hispanic 2. Black, Non Hispanic 3. Hispanic or Latino 4. Other or unknown |
42 8 12 8 4 |
30 3 6 5 3 |
12 5 6 3 1 |
|
| Treatment naïve | 48 | 34 | 14 | |
| Prior treatment | 26 | 13 (27.7%) | 13 (48.1%) | 0.076 |
| Non-cirrhotic | 46 | 32 | 14 | |
| Cirrhotic | 28 | 15 (31.9%) | 13 (48.1%) | 0.166 |
| Genotype 1a 1b 1 |
12 14 48 |
6 7 34 |
6 7 14 |
|
| Co-morbidity Charlson Score | 1-2: 19 (25.3%) 3-4: 27 (36.5%) 5 +: 28 (37.3%) |
1-2: 14 (29.8%) 3-4: 12 (25.5%) 5 +: 21 (44.7%) |
1-2: 5 (18.5%) 3-4: 15 (55.5%) 5 +: 7 (25.9%) |
|
| Mean Charlson Score | 4.45 | 3.89 | 0.379 | |
| Overall SVR | 41/74 (55.4%) | 29/47 (61.7%) | 13/27 (48.1%) | 0.257 |
| Boceprevir SVR | 26/49 (35.1%) | |||
| Telaprevir SVR | 15/25 (60.0%) | |||
| ED/Hospital AE | 28/47 (59.6%) | 9/27 (33.3%) | 0.030 | |
| Discontinued | 6/47 (12.8%) | 5/27 (18.5%) | 0.460 | |
| Hospitalization during rx | 8/47 (17.0%) | 3/27 (11.1%) | 0.490 |
Psychiatric and Substance Use Disorder (SUD) diagnoses*.
| Psych diagnosis | Primary Care, n=47 (% of cohort) |
Hepatology, n=27 (% of cohort) |
SUD diagnosis | Primary Care, n=47 (% of cohort) |
Hepatology, n=27 (% of cohort) |
|---|---|---|---|---|---|
| PTSD | 30.4% | 24.0% | Alcohol use/dependence | 58.7% | 32.0% |
| Depression | 37.0 | 40.0 | Cocaine dependence | 30.4 | 4.0 |
| Depressive disorder | 19.6 | 8.0 | Cannibis abuse/dependence | 23.9 | 4.0 |
| Schizoaffective | 6.5 | 0.0 | Amphetamine abuse | 34.8 | 12.0 |
| Mood disorder | 6.5 | 4.0 | Tobacco use disorder | 45.7 | 32.0 |
| Adjustment disorder | 2.2 | 8.0 | Opiod type dependence | 19.6 | 12.0 |
| Generalized anxiety disorder | 4.3 | 4.0 | Drug abuse, IV | 0 | 4.0 |
| Panic disorder | 2.2 | 0.0 | Cannibis dependence remission | 2.2 | 4.0 |
| Psychotic disorder | 4.3 | 0.0 | Polysubstance dependence | 4.3 | 0 |
| TBI, cognitive disorder | 4.3 | 0.0 | Hypnotic sedative anxiolytic dependence | 4.3 | 0 |
| Paranoid Schizoaffective | 2.2 | 4.0 | Heroin dependence | 2.2 | 0 |
| ADHD | 0 | 4.0 | |||
| Psychotic disorder | 2.2 | 0 | |||
| Pain disorder with psych disorder | 2.2 | 0 | |||
| Social phobia | 4.3 | 0 | |||
| Amphet-induced psychosis | 2.2 | 0 | |||
| Bipolar disorder | 2.2 | 0 | |||
| Patients with single psych diagnosis (n, %) | 18/47 (38.3%) | 8/27 (29.6%) | Patients with single SUD diagnosis (n, %) |
7/47 (14.9%) | 11/27 (40.7%) |
| Patients with >2 psych diagnoses (n, %) | 17/47 (36.2%) | 7/27 (25.9%) | Patients with >2 SUD diagnoses (n, %) |
28/47 (59.6%) | 6/27 (22.2%) |
| Patients with no psych diagnosis (n, %)* | 12/47 (25.5%) | 12/27 (44.4%) | Patients with no SUD diagnosis (n, %) |
12/47 (25.5%) | 10/27 (37.1%) |
& Patients without psychiatric or SUD diagnoses included 5/47 (10.6%) in the Primary Care cohort and 5/27 (18.5%) in the Hepatology cohort.
Primary care providers treated 47 patients (32% cirrhotic) vs. 27 patients treated by hepatologists (48% cirrhotic). Overall, early treatment termination due to adverse events occurred in 5.3%, and treatment termination for virologic non-response occurred in 21.3% patients. The percentage of patients that completed 0-19 weeks, 20-28 weeks, 29-36 weeks, and greater than 36 weeks of antiviral treatment was 25.3%, 37.3%, 9.3%, and 26.6%, respectively. The percentage of patients completing antiviral treatment with Primary Care and Hepatology providers are indicated in Fig. (1). Overall, patients in the Primary Care provider cohort demonstrated a trend for better adherence to planned duration of therapy. Compliance with recommended treatment duration was very high in the overall cohort at 93.2%.
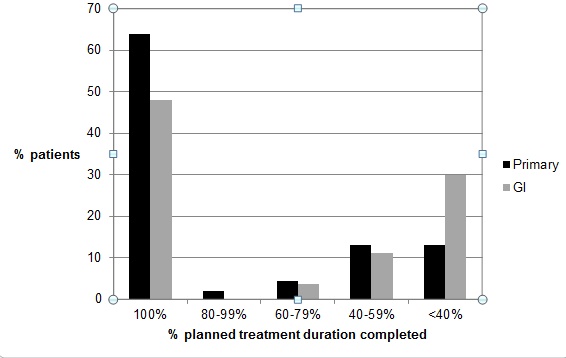
Percent of planned treatment duration achieved by patients treated by Primary care providers (Primary) vs. hepatologists (GI). Primary care providers treated 47 patients (32% cirrhotic) vs. 27 patients treated by hepatologists (48% cirrhotic).
Final SVR rates observed are listed in Table 1 and Fig. (2). The overall SVR rate was 54.6% in the entire cohort. As expected, the SVR rate was higher in non-cirrhotics vs. cirrhotics (65.2 vs. 39.2%). SVR rates were higher in patients primarily cared for by Primary Care providers (61.7%) compared with Hepatology providers (44.4%), likely due to the higher incidence of cirrhosis in the latter group. SVR results for patient subgroups are listed in Table 3.
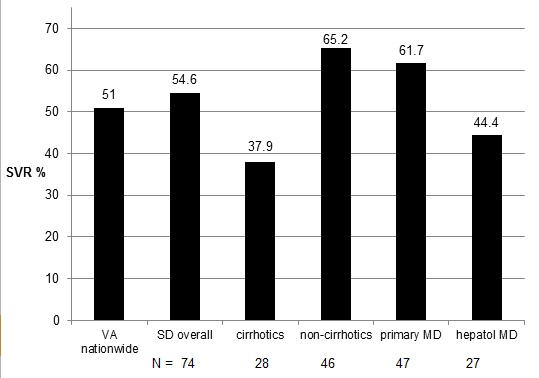
SVR rates with genotype 1 patients treated with DAA therapy. (National VA treatment results with first generation DAA ref. [17]).
SVR results for patient subgroups.
| Patient Characteristics | Total treated (n=74) SVR (n,%) |
Boceprevir (n=49) SVR (n,%) |
Teleprivir (n=25) SVR (n,%) |
|
|---|---|---|---|---|
| Primary care provider • Total • Non-cirrhotic • Cirrhotic • Rx naïve • Prior failure |
n=47 n=32 n=15 n=34 n=13 |
29 (61.7%) 20 (62.5%) 9 (60.0%) 22 (64.7%) 7 (53.8%) |
29 (61.7%) 22 (68.7%) 7 (46.7%) 16 (47.0%) 1 (7.7%) |
18 (38.3%) 8 (25.0%) 10 (66.7%) 6 (17.6%) 6 (46.1%) |
| Hepatology provider • Total • Non-cirrhotic • Cirrhotic • Rx naïve • Prior failure |
n=27 n=14 n=13 n=14 n=13 |
13 (48.1%) 10 (71.4%) 3 (23.1%) 9 (64.3%) 4 (30.8%) |
20 (74.1%) 11 (78.6%) 9 (69.2%) 6 (42.8%) 3 (23.1%) |
7 (25.9%) 3 (21.4%) 4 (30.8%) 3 (21.4%) 1 (7.7%) |
Adverse events (ED or hospital admission, treatment discontinuation).
| Patient Characteristics | Total # adverse events | Reasons for ED visits, most frequent | Reasons for Admissions | |
|---|---|---|---|---|
| Primary care provider | n=47 | 48 ED visits, 8 hospitalizations (ED were visits for 27 patients) and 1 death (sudden death due to alcohol relapse on antiviral treatment) | Pain, altered mental status, fatigue, nausea, emesis, hypotension, infection | Involuntary psych hold, dyspnea, psychosis, chest pain, nausea emesis acute kidney injury, pneumonia, hypotension |
| Hepatology provider | n=27 | 16 ED visits, 3 hospitalizations (ED visits were for 9 patients) and 0 deaths | Pain, cough, altered mental status, dyspnea | Dyspnea, pre-syncope |
Multiple adverse events related to the antiviral therapy were observed and managed by the providers (Table 4). During antiviral treatment, serious adverse events requiring urgent emergency room visits occurred in 27 (54%) patients in the primary care cohort, with a total of 48 visits. Serious adverse events requiring ED visits occurred in 9 (33.3%) patients treated by Hepatologists, with a total of 16 visits. During antiviral therapy, 11 patients required hospitalization, 8 from the Primary care group and 3 from the Hepatologist care group. Of the cirrhotic patients, 12 (42.8% of cirrhotics) were seen in the ED with a total of 22 visits, of these patients 7 required hospitalization. Of the non-cirrhotic patients, 24 (52.1% of non cirrhotics) were seen in the ED with a total of 42 visits, of these patients 5 required hospitalization. One patient died during the course of treatment in the Primary Care cohort, from a relapse to alcohol abuse and a presumed sudden cardiac death event. Despite multiple adverse events, early treatment termination due to adverse events occurred in only 5.3%. In contrast, treatment termination for virologic non-response was observed in 21.3% patients.
Multivariate analyses tested whether the incidence of SVR, treatment discontinuation, or adverse events (ED or hospitalization) differed between the Primary care and Hepatology cohorts after controlling for cirrhosis (yes/no), Charlson score, and treatment history using logistic regression. For SVR and treatment discontinuation, no significant differences were found between clinics. For adverse events, there was a trend toward more ED visits/hospitalizations in the primary group after controlling for covariates (p = 0.08).
DISCUSSION
This primary care and mid-level provider-based clinic with co-located hepatologists and one psychiatrist demonstrated high patient volumes and access. DAA antiviral therapy administered by primary care providers achieved SVR rates of 61.7%, approaching published clinical trials, and exceeding the overall SVR rate reported for these first generation DAA treatments in the VA system [17, 18]. These data illustrate the potential for a collaborative clinic with effective state of the art therapies for patients with high prevalence of comorbidities. Similar models may be useful for expanding access to effective HCV care in other clinic and hospital settings.
Available since 2001, pegylated interferon combined with ribavirin resulted in 41-44% sustained viral response (SVR) rates for genotype 1 in clinical trials [19, 20]. Real-world SVR rates for hepatitis C therapy are significantly lower, and in the VA system averaged approximately 26% in genotype 1 patients [21]. In 2011 two new HCV protease inhibitors, Telaprevir and Boceprevir, for use in combination with the previous standard of care pegylated interferon alfa and ribavirin (PR) were approved. These drugs represent a new category known as direct acting antivirals (DAA). Phase III clinical trials demonstrate improvement in SVR rates for genotype 1: 68-75% for treatment-naïve patients, and 53% for patients that have failed previous PR treatment [6, 7, 22, 23]. Results from actual VA clinics indicate that overall SVR rates of 51% were achieved with Telaprevir and Boceprevir-based regimens for patients (42.7% among patients with cirrhosis, 56.8% among treatment-naive patients) [17, 18]. Our data indicate that a primary care based clinic meets or exceeds the overall reported SVR rates for current DAA therapies, with no significant differences in SVR in patients cared for by primary care vs. hepatology providers.
Our data indicated a very high incidence of adverse events associated with DAA treatment. The adverse events on treatment that resulted in ED visits and hospitalizations tended to be higher in patients with primary care vs. hepatology providers. This may be explained in part by the fact that the mean comorbidity score in patients cared for by primary care tended to be higher than patients care for by hepatology providers, and the fact that the clinic had no mechanism to provide same-day service for patients calling with significant side effects, and therefore they tended to be referred to the emergency department. The data are limited by the relatively small numbers of patients; however these data certainly illustrate the difficulties of using DAA with pegylated interferon and ribavirin treatments, especially in patients with significant medical co-morbidity or cirrhosis. Side effect management with newer antiviral treatments without interferon will be much less of an issue and would favor treatment provided by primary care providers.
A similar model of expanded primary care involvement in hepatitis C care is the ECHO model [24]. This is a collaborative care model for involvement of primary care providers linked by a weekly telemedicine conference with hepatitis C specialists for the purpose of providing help for primary care providers related to specific patients and also to provide ongoing education related to hepatitis C treatment. Expanding or enabling primary care providers to treat patients with hepatitis C may be difficult in the absence of incentives, especially for primary care practices that may be overwhelmed by existing demands. The administrators of a managed care or group practice system would have to allow for incentives for primary care to deliver HCV treatment, based on recognition for the need to improve access to care for their patients in order to achieve better long term outcomes and lowered medical care costs. In our case this was negotiated with the administrators of the primary care clinics to allow dedicated time for this activity. Incentives for the primary care providers in our case included increased job satisfaction and team building. For the ECHO model increased job satisfaction and access to continuing medical education credit were cited as incentives.
Based on data to date, providers would have multiple options of how to adopt a primary care based approach to hepatitis C care. One option is to collaborate with existing HCV clinics to strengthen and increase the interdisciplinary aspect of the clinic, as was the model for the current study. This would be most applicable to managed care type organizations and large group practices or accountable care organizations. Alternatively, the provider could provide HCV care in the context of their own clinic, provided they set up virtual collaborations with specialists who could assist if needed, along the lines of the ECHO model as described above.
HCV is a chronic illness affecting patients with multiple co-morbidities, which can affect both patient and provider willingness to undergo antiviral treatment. Psychiatric and substance use disorders are common in patients with hepatitis C, and continue to represent barriers to obtaining DAA treatment even in the interferon-free era. Currently in most states Medicaid precludes the use of DAA in patients with ongoing substance abuse [25]. Recently, 45% of consecutive patients attending a HCV clinic at one VA medical center were considered to be poor candidates for interferon-free DAA treatment due to psychiatric and substance use issues that could affect compliance [26]. Team-based attempts to integrate a variety of services to address complex interrelated health problems have been shown to maximize adherence and outcomes in primary care studies related to substance abuse, depression, and HIV management. These “integrated care” models include the elements of multi-disciplinary teams, co-location, increased communication, and shared patient protocols and goals of treatment. The primary-care based HCV clinic was organized using these principals and may have contributed to the successful outcomes. Interestingly, patients treated by the primary care providers in the current study tended to have more comorbidities, especially related to substance use and psychiatric disease. This is likely due to chance from the relatively small number of patients reported; however it does strengthen the conclusion that the primary care providers are able to provide excellent care for the patients. The patients who were referred to the specialists were primarily patients with cirrhosis, and not necessarily the most complicated overall. Integrated care for hepatitis C has been found to be effective in a randomized trial in the context of a specialty clinic using mid-level providers in a collaborative environment including hepatology and psychiatric practitioners [27]. The current model expands this to include primary care providers for first-line patient management. Given the extensive co-morbidities of HCV patients, further efforts to incorporate primary care providers into HCV treatment clinics should build on these integrated care concepts.
Rapidly evolving DAA treatments herald the potential for a much wider uptake and acceptance of HCV antiviral treatment. Currently, the large majority of current antiviral treatment consists of the interferon-free DAA regimens Viekira pac (ombitasvir, dasabuvir, paritaprevir, and ritonavir) and Harvoni (sofosbuvir and ledipasvir) with or without ribavirin. These are much simpler regimens with very few side effects, and therefore are much easier to manage. The development of these regimens enables many more HCV patients to become treatment candidates, which greatly increases the clinical demand and strengthens the case for primary care involvement in HCV care. In addition, the lack of significant side effects will improve the ability and interest of primary care providers in participating in HCV care. The study presented demonstrates the feasibility and the efficacy of a primary care based half day clinic organized using a collaborative or integrated care model, and should be considered for clinics seeking to expand care for patients with chronic hepatitis C.
LIST OF ABBREVIATIONS
| DAA | = Direct-acting antiviral |
| HCV | = Hepatitis C virus |
| PEG | = Pegylated interferon alfa |
| RBV | = Ribavirin |
| VA | = Veterans Affairs |
DISCLOSURES
Samuel B. Ho, MD: research and grant support: Genetech, Inc., Gilead, Inc, Prime Education, Inc. Other authors: nothing to disclose.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Grant Support: Funding provided by VA HSR&D grant IIR-07-101-3 , VA HIV/HCV QERI program, and the Research Service of the Department of Veterans Affairs.


