All published articles of this journal are available on ScienceDirect.
Cognitive Performance Deficits and Dysgraphia in Alzheimer’s Disease Patients
Abstract
Introduction:
Agraphia or dysgraphia, observed often in early AD, encompasses a progressive disorganization and degeneration of the various components of handwriting.
Methods:
Deficits in writing ability, dysgraphia, and the relationship with other measures of cognitive decline were studied in a group of 30 patients, originating from the Lazio region, Rome, Italy, presenting a moderate to relatively severe stage of Alzheimer’s disease (AD). Extent of dysgraphia and cognitive performance was compared with a matched group of healthy controls selected from the same region.
Results:
Several markedly strong relationships between dysgraphia and several measures of cognitive performance in AD patients were observed concomitant with consistent deficits by this patient sample in comparison with the matched group of healthy control subjects were obtained. Additionally, several measures of loss of functional integrity, MMSE, ADL and IADL, were found to be associated with both dysgraphia and impairments in cognitive performance.
Conclusion:
The present results are discussed from the notion of affected brain regions underlying functions in cognition, language and motor domains that are disturbed in AD.
INTRODUCTION
According to the World Health Organization (WHO), the number of people affected by dementia was 35.6 millions [1] in the year 2011. This figure is destined to increase at an alarming rate; since the aging of the population [2, 3] has an influence both on the incidence and on the prevalence of this syndrome, it has been estimated that by the year 2050 the number of people affected will be 115.4 million [4]. The pathology of dementia is associated with memory loss, loss of orientation, inability to focus attention, loss of speech. The compromised learning capability, ability to calculate, and impaired judgment are often accompanied or preceded by behavioral disturbances, impaired emotional regulation, and lack of motivation [4]. In Western industrial nations, Alzheimer’s disease (AD) represents the most common form of dementia [5, 6], approximately 80% of cases [7], occupying fourth place among the causes of death (after heart disease and circulatory disorders, cancer and cerebral hemorrhage); the secondary forms of dementia, such as vascular dementia, Lewy bodies dementia and the front temporal dementia, appear to be less common [8]. In common, the clinical symptoms presented by AD patients are characterized by memory impairment and at least a cognitive alteration, i.e. aphasia, apraxia, agnosia, or an alteration in executive functions [9], all of whichcomplicates the derivation of a differential diagnosis [10]. Even if determined as 'possible' or 'probable' with a percentage of 80%, after tests, blood tests, urine test and spinal cord analysis, or imaging tests such as computed tomography (CT), magnetic resonance imaging (MRI) and through new imaging techniques, diagnosis becomes finally “accepted” only after post-mortem examination of brain tissue [11, 12]. In this regard, all evidence contributing to loss of function in motor, cognitive and emotional domains ought to be valuable. The neurological damage and cognitive dysfunction, including loss of memory, difficulties of written and oral communication, etc., cause several problems of daily living [13, 14], due to which patients are unable to cater their own interests thereby ensuring cumulative deterioration in their quality of life [15].
As early as 1907, Alzheimer [16] had observed in these patients abnormal graphic gestures which indicated that hand writing is not constituted by a unitary process, but that it requires a coordination of linguistic and visual-spatial of the individual [17, 18] reflecting brain damage in different associative areas, such as parietal, temporal, occipital and frontal regions [19], subsequently diagnosed in AD patients [20]. Lambert et al. [21] have demonstrated a wide variety of agraphia syndromes, including a far from negligible number of patients with selective damage to one of the central or peripheral components, as well as patients with multiple writing impairments. A positive correlation was observed between the severity of the dementia and spelling/writing measures (lexical and allographic). Agraphia or dysgraphia, observed in early AD [22], encompasses a progressive disorganization and degeneration of the various components of handwriting [23]; these include the complexity of the structure of sentences [24], the diversity and the accuracy of words used [25], punctuation [26], organization [25], the production of grammatically incorrect sentences [27, 28], the length of the sentences [27], the amount of written information [28], the morphology of the letters [27] and spelling [29], graphic and spatial layout of letters and their arrangement in texts [18]. Fukui and Lee [30] examined the possibility that agraphia/dysgraphia may be an early sign of degenerative dementia, reporting the concurrent or subsequent emergence of non-fluent aphasia, ideomotor apraxia, executive dysfunction and asymmetric akinesic-rigid syndrome; these observations implicate degenerative processes involving the parietal-occipital-temporal regions, basal ganglia and striato-frontal projections. It has been observed that that writing impairment is heterogeneous within the AD population, but nevertheless, there are certain aspects of the writing process that are more vulnerable than others and may present diagnostic signs [31]. The identification and staging patterns of writing impairment/deficits during different phases of AD may facilitate the understanding of disease progression and present conditions for the development of relevant interventions.
The purpose of the present study was to examine the relationship between cognitive impairment and the performance of handwritten scripts presented as ‘letter-writing’ to a close relative by AD patients, as oscillations of the symptoms phase, and in a matched group of healthy controls. It is possible that in graphic expression even by patients diagnosed with moderate to relatively severe AD there remains some residual capacity for understanding and intention that may be expressed. Thus, a major focus of this study was to reveal, through correlational analyses, the implication of progressive agraphia in degenerative dementia since patients with moderate to relatively severe stage were analyzed. Additionally, comparisons between the AD patients and healthy controls were analyzed also. Concurrently, the relationship between measures of functioning, Mini-Mental State Examination (MMSE), Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) with dysgraphia and assessments of cognitive performance was assessed also.
METHODS AND MATERIALS
Participants
There were 30 patients who were selected to participate in the study, 12 male and 18 female AD patients who met both the inclusion criteria (see Table 1) and the exclusion criteria, i.e. absence of other neuropsychiatric disorders. The diagnosis was based on normal or nonspecific EEG and lateral, occipital brain atrophy on CT brain with documented progression after serial observations on the cognitive tests and routine blood tests that aimed at excluding the presence of other medical conditions that can justify dementia.
Demographic, neuropsychological and clinical characteristics of each of the AD patients in the present study.
| Patient No. | Education (yrs) | Age (yrs) | Sex (M/F) | cognitive deficits | MMSE1 | ADL2 | IADL3 | debut(yrs)4 | Domicile |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 73 | F | aprax, aphas | 16(13,7) | 4 | 4 | 3 | relatives |
| 2 | 6 | 93 | F | aprax, aphas, agnos | 13 | 4 | 2 | 6 | caregivers |
| 3 | 10 | 91 | F | aprax, aphas, agnos | 12 | 4 | 4 | 5 | relatives |
| 4 | 7 | 90 | F | aprax, aphas, agnos | 11 | 2 | 2 | 7 | relatives |
| 5 | 15 | 87 | F | apraxia, aphasia | 15(15,3) | 4 | 4 | 6 | relatives |
| 6 | 8 | 77 | F | apraxia, aphasia | 16(15) | 5 | 4 | 5 | caregivers |
| 7 | 7 | 78 | F | apraxia, aphasia | 14(12,3) | 4 | 4 | 4 | relatives |
| 8 | 12 | 79 | F | aprax, aphas, agnos | 13(11,3) | 4 | 4 | 5 | caregivers |
| 9 | 14 | 79 | F | apraxia, aphasia | 15(13,3) | 4 | 4 | 4 | caregivers |
| 10 | 8 | 80 | F | apraxia, aphasia | 17(16,7) | 5 | 4 | 3 | relatives |
| 11 | 14 | 77 | F | aprax, aphas, agnos | 14(12,3) | 4 | 4 | 4 | relatives |
| 12 | 13 | 77 | F | aphasia, apraxia | 17(15,3) | 4 | 4 | 3 | caregivers |
| 13 | 12 | 81 | M | apraxia, aphasia | 15(14,7) | 4 | 4 | 4 | caregivers |
| 14 | 16 | 85 | M | aprax, aphas, agnos | 12(12,3) | 4 | 2 | 5 | relatives |
| 15 | 15 | 83 | M | agnos, aprax | 11(10,1) | 4 | 2 | 6 | relatives |
| 16 | 15 | 83 | M | aphasia, apraxia | 14(13,1) | 4 | 2 | 5 | caregivers |
| 17 | 8 | 80 | M | apraxia, aphasia, | 17(16,7) | 5 | 2 | 3 | relatives |
| 18 | 17 | 80 | M | apraxia, aphasia, | 14(13,1) | 4 | 4 | 4 | relatives |
| 19 | 6 | 94 | M | agnosia, apraxia | 16 | 4 | 4 | 2 | relatives |
| 20 | 8 | 93 | M | apraxia, agnosia, aphasia | 14 | 4 | 4 | 5 | caregivers |
| 21 | 10 | 92 | F | agnosia, apraxia | 12 | 4 | 4 | 4 | relatives |
| 22 | 7 | 91 | F | aphasia, apraxia | 15 | 4 | 4 | 5 | relatives |
| 23 | 18 | 80 | M | aphasia, apraxia | 17(16,1) | 4 | 2 | 3 | relatives |
| 24 | 10 | 78 | M | apraxia, | 17(16) | 5 | 4 | 4 | relatives |
| 25 | 12 | 77 | M | apraxia, aphasia | 17(16) | 4 | 4 | 4 | caregivers |
| 26 | 10 | 76 | F | aphasia, apraxia | 16(15) | 5 | 4 | 4 | relatives |
| 27 | 13 | 79 | F | aphasia, apraxia | 18(16,3) | 5 | 4 | 3 | relatives |
| 28 | 16 | 83 | M | agnosia, aphasia | 12(11,1) | 2 | 2 | 6 | relatives |
| 29 | 8 | 85 | M | aphasia, apraxia | 12(12,8) | 3 | 2 | 5 | caregivers |
| 30 | 7 | 91 | F | agnosia, apraxia, aphasia | 11 | 4 | 2 | 6 | caregivers |
1MMSE=mini-mental state examination [33] [normal level score=30 points], modified by age and education (numbers in brackets); 2ADLs=Activities of Daily living [34] [normal level=6/6 for both males and females]; 3IADLs=Instrumental activities of daily living [35] [normal level=8/8 for both males and females]; AD=Alzheimer’s disease; aphasia=aphas; agnos=agnosia; apraxia=aprax; 4Debut: numbers of years elapsed since first indication of disorder.
Assessment test of cognitive functions consisting of 14 items (see below), modified version of the Folstein MMSE (1975), adapted specially for patients presenting severe AD. An imaginary example of a patient is provided below.
| Personal Details | Score |
|---|---|
| 1. What is your name ? | 0-3 |
| 2. When were you born ? | 0-2 |
| 3. Who am I? | 0-2 |
| Temporal Orientation | Score |
| 9. What year is it? | 0-1 |
| 10. What season are we in? | 0-1 |
| 11. What month is it? | 0-1 |
| 12. What is the date today? | 0-1 |
| 13. What day of the week is today? | 0-1 |
| 14. Is it day or night ? | 0-2 |
| Spatial Orientation | Score |
| 4. What country are we in? | 0-1 |
| 5. In which Italian region are we in? | 0-1 |
| 6. In which city are we in? | 0-1 |
| 7. What floor are we at? | 0-1 |
| 8. Where are you | 0-2 |
Total score, PQ = 20 points.
T-test values between PQ1-PQ2 scores (means±SD), and Pearson product moment correlational analyses between (i) PQ1 and writing-time (min), (ii) writing-time (min) and DΔ over 10 days of testing (Days 1, 3, 5, 7, 9, 11, 13, 15, 17 and 19).
| Test Days | PQ1 | PQ2 | t-values | R (i) | R (ii) |
|---|---|---|---|---|---|
| 1 | 11.9±4.8 | 4.0±1.9 | 8,25* | 0.802* | 0.905* |
| 3 | 10.5±4.3 | 4.1±2.3 | 7,11* | 0.785* | 0.669* |
| 5 | 9.9±5.1 | 4.8±3.0 | 4,71* | 0.603○ | 0.680* |
| 7 | 9.5±4.6 | 5.2±2.6 | 4,39* | 0.644○ | 0.755* |
| 9 | 11.8±3.1 | 5.7±2.1 | 9,04* | 0.732* | 0.588○ |
| 11 | 8.9±4.4 | 5.0±2.8 | 4,04* | 0.556▲ | 0.646○ |
| 13 | 9.3±4.3 | 5.5±2.7 | 4,08* | 0.688* | 0.542▲ |
| 15 | 8.9±4.4 | 5.1±2.9 | 3,93* | 0.498● | 0.643○ |
| 17 | 9.5±4.0 | 5.4±3.1 | 4,42* | 0.767* | 0.547▲ |
| 19 | 9.5±5.3 | 4.5±3.4 | 4,26* | 0.871* | 0.576○ |
*t-tests (df=58) p < 0,0001.●p < 0.005; ▲p < 0.002; ○p < 0.001.
Pearson product moment correlations between the functional measures, ADL, IADL and MMSE, and the assessments of cognitive performance, PQ1, DΔ and PQ2, in the 30 patients.
| PQ1 | D∆ | PQ2 | MMSE | Writing-Time | |
|---|---|---|---|---|---|
| ADL | 0.394* | 0.223ns | 0.512** | 0.611*** | 0.457** |
| IADL | 0.471** | 0.368* | 0.124ns | 0.378* | 0.489** |
| MMSE | 0.711*** | 0.650*** | 0.649*** | 0.708*** | |
| D∆ | 0.850**** |
ADLs = activities of daily life; IADLs = instrumental activities of daily life; MMSE = mini-mental state examination.
*p < 0.05, **p < 0.01, ***p < 0.0005, ****p < 0.000001, two-tailed tests.
Pairwise differences (Student’s t-test) between AD patients and controls for (i)4A: writing-time (min), (ii)4B: dΔ, over 10 days of testing (Days 1, 3, 5, 7, 9, 11, 13, 15, 17 and 19).
| A | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | Day 15 | Day 17 | Day 19 |
|---|---|---|---|---|---|---|---|---|---|---|
| t-values: | 8.80* | 12.32* | 8.47* | 10.49* | 14.49* | 12.87* | 13.99* | 12.41* | 9.00* | 9.00* |
| AD | ||||||||||
| Mean | 8.73 | 8.13 | 8.53 | 5.63 | 8.03 | 5.33 | 5.57 | 5.37 | 6.43 | 7.40 |
| SD | 6.65 | 4.90 | 7.10 | 5.35 | 5.97 | 5.23 | 5.84 | 5.60 | 5.49 | 7.39 |
| Control | ||||||||||
| Mean | 19.60 | 19.53 | 19.63 | 19.70 | 19.63 | 19.60 | 19.57 | 19.80 | 19.33 | 19.70 |
| SD | 1.25 | 1.28 | 1.00 | 1.15 | 1.00 | 1.30 | 1.17 | 0.76 | 1.52 | 1.15 |
| B | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | Day 15 | Day 17 | Day 19 |
| t-values: | 8.68* | 11.20* | 6.90* | 7.14* | 12.06* | 7.65* | 6.50* | 7.02* | 7.73* | 9.00* |
| AD | ||||||||||
| Mean | 7.83 | 6.33 | 5.10 | 4.30 | 6.17 | 3.87 | 5.57 | 3.80 | 4.10 | 4.93 |
| SD | 4.85 | 3.03 | 3.95 | 3.19 | 2.72 | 2.62 | 5.84 | 2.86 | 2.71 | 2.89 |
| Control | ||||||||||
| Mean | 0.10 | 0.10 | 0.10 | 0.07 | 0.13 | 0.13 | 0.10 | 0.10 | 0.20 | 0.13 |
| SD | 0.55 | 0.31 | 0.40 | 0.64 | 0.35 | 0.51 | 0.40 | 0.40 | 0.55 | 0.43 |
* versus control group, Student’s t-test (df=1, 58), p < 0.0001.
Patients were excluded if they had presented a history of known or suspected cerebrovascular disease, focal neurological signs or on brain imaging, alcohol misuse, head trauma, significant psychiatric history preceding the current diagnosis or other major physical illness. The ages of the participants varied between 73 to 94 years of age. (mean age: 83.06, SD: 6.15). All of the patients were presenting symptoms that indicated a diagnosis of AD from moderate level to relatively severe (see Table 1). They were diagnosed according to the NINCDS-ADRDA criteria [32] and tothe DSM-IV diagnostic reference. All of these diagnoses were confirmed and verified in 26 patients by resident neurologists at the Department of Neurology at the hospital (Gemelli University Polyclinical-service neuropsychology, Roma and UVA (Alzheimer Evaluation Unit) ASLRMF and UVA (Alzheimer Evaluation Unit) ASLRMD, and in 4 patients from the Department of Neurology and Psychiatry, Sapienza Hospital, Rome) in the Lazio (Rome, Italy) region. The clinical characteristics of the participants in the study are presented in Table 1. The mean length of time spent upon education by the 30 patients was 11.06 years (SD ± 3.6 years). A considerable amount of time (regular meetings during 3 months) was invested in each of the patients in order to promote a relationship of trust and understanding, as well as to reduce stress factors [6] that may affect patients’ mood and attentiveness, or, more seriously, induce dysfunctional behaviors that may be taken for psychiatric incapacity, difficulty to recognize persons, or loss of cognition of time and places through being asked to work with some person whom they did not trust. All the procedures that were adopted according to discussions and meetings with nearest relatives in order to obtain the consent of the patients as well as those relatives (in those cases were the latter were their caregivers/legal representatives) according to the legal practices. The control group of age- and education-matched healthy senior citizens was chosen as individuals that were not in any way influenced by AD and whom presented the following characteristics: mean age 82.73 years (SD ± 5.7 years). The mean amount of time spent upon education by the healthy controls was 12.8 years (SD ± 4.04 years).
AD Diagnosis: All the patients evidenced lower performance in the standard tests of neuropsychological assessment that were administered. The cognitive profiles presented by these patients expressed cognitive impairments that were widespread and related to a severe dementia syndrome of a progressive nature that was linked to a degenerative dementia of the Alzheimer type. On the basis of the neuropsychological tests and clinical observations this group of patients was classified at the moderate to relatively severe stage of AD.
Mini-mental State Examination (MMSE) presents a brief 30-point questionnaire test to screen for cognitive impairment and dementia [33]. It estimates the severity of disorder and follows the course of cognitive changes in an individual over time, thereby allowing effective monitoring of an individual's response to treatment. Table 1 presents the individual scores of each of the patients. It will be noted that these scores range from 11 to 18 which implies that the patients express a moderate level of AD disorder. Healthy control individuals scored at 30 points.
Activities of Daily Living (ADLs) offers an instrument that measures everyday behaviors necessary for normal functioning on a daily basis [34]. Under normal conditions, individuals must invest a certain amount of time taking care of their personal care and hygiene in order to promote their own health and to a sufficiency of independent initiative and capability.
Instrumental Activities of Daily Living (IADLs) offers an instrument that measures those behaviors that are linked to an independent lifestyle. The instrument has been found to be useful for evaluations of individuals presenting early-stage (or moderate stage) disorders: it has been found applicable for ascertaining both disorder extent and determination of individual capacity for self-care and management [35].
Neurological Data for structural neuro-imaging analysis was obtained from computerized axial tomography (CAT) whereas magnetic resonance imaging (MRI) data were not available. The CAT data were used only for confirmation of diagnosis and were not judged to be of sufficient quality to permit an analysis to combine with the measures of cognitive performance.
Cognitive Performance Assessment: Graphia and Memory Tests
Testing Material. A tablet (writing-pad) was used for writing text or drawing figures, preferably the vergatina type (flimsy type, typing paper or tissue type or absorbent in order to avoid false interpretations of the writings to analyze. A ballpoint pen was used throughout. The patients were invited to sit in a comfortable position.
Procedure
In order to test the cognitive performance of AD patients and healthy controls, as well as their spatial and time orientation and their residual capacity, a standard collection of 14 simple questions were presented to the patients and controls. The questions were derived and modified from the MMSE 1 and 2 items to cover the temporal and spatial orientation. These questions were designed and presented in a form that could be utilized by any General Practitioner (GP) in order to document the level of cognitive functioning of each patient. For each correct answer one point was attributed in proportion to the difficulty of the question (see Table 2). The total sum from each test session was represented by PQ: the initial session result designated PQ1. Following this, each patient was then invited to write a letter to a close relative. On consecutive days of testing patients were invited to write to either the same relative or another one. Using a chronometer to establish length of writing-time (min.), the letter-writing task was interrupted when it seemed that the text produced by the patient was substantially (pathologically) confused, both with regard to spatial disorientation as well as for a sudden lack of readability, disjointedness and incompleteness in meaning (see Fig. 1). After this, the number of minutes (min) that had been reached for each single patient was registered. For Patient X (see above) the whole procedure involving the letter-writing graphia task was interrupted after 10 minutes since the patient continued to write, but in an incomprehensible manner. After having terminated the writing session, the questionnaire was presented again to the patients with the scores thus provided constituting PQ2.

Depicts the performance of Patient X during PQ1 followed by the letter-writing test followed by PQ2. The quality of handwriting, coherence and comprehension is taken into account before deciding to terminate the writing session.
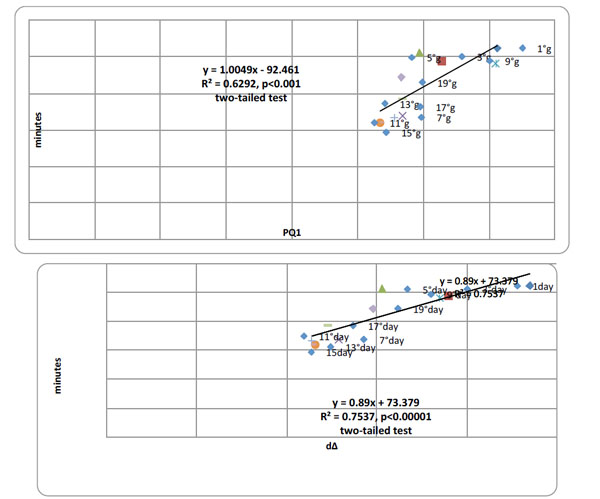
Top panel. Pearson product moment correlational analyses between PQ1 and writing-time (min) over all 10 days of testing (Days 1, 3, 5, 7, 9, 11, 13, 15, 17 and 19) summated over all 30 patients, Pearson r=0.793 (df=30), p < 0.000001. Bottom panel. Pearson product moment correlational analyses between writing-time (min) and dΔ over 10 days of testing (Days 1, 3, 5, 7, 9, 11, 13, 15, 17 and 19) summated over all 30 patients, Pearson r=0.868 (df=30), p < 0.0000001, two-tailed tests.
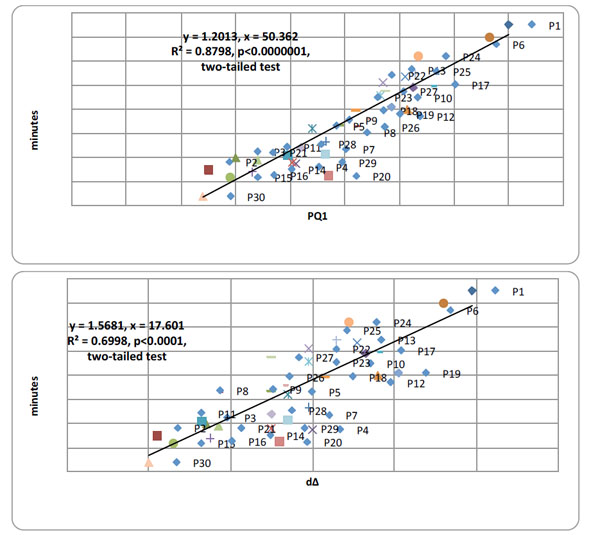
Top panel. Pearson product moment correlational analyses between PQ1 and time writing (min) over all 30 AD patients summated over all 10 days of testing, Pearson r=0.938 (df=12), p <0.000001. Bottom panel. Pearson product moment correlational analyses between time writing (min) and dΔ over all 30 AD patients summated over all 10 days of testing, Pearson r=0.829 (df=12), p < 0.0000001, two-tailed tests.
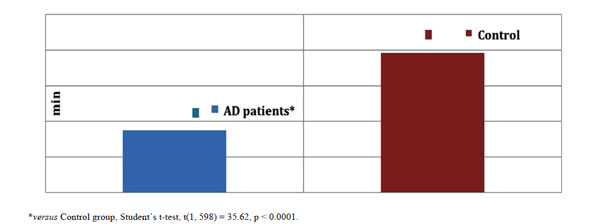
The amount of time spent writing (min) by AD patients and healthy controls expressed as means ± SD in the graphia test, summated over all 12 days of testing.
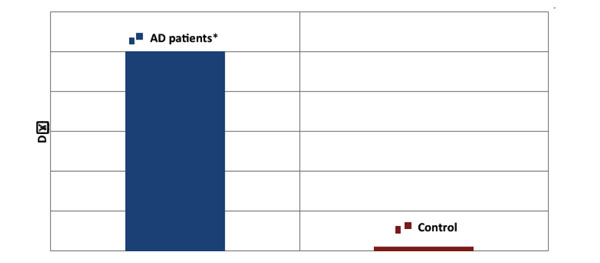
The difference (DΔ) between cognitive performance in PQ1 and PQ2 by AD patients and healthy controls expressed as DΔ means ± SD, summated over all 10 days of testing. *versus Control group, Student’s t-test, t(1, 598) = 24.35, p < 0.0001.
As a test for patients presenting dementia this set-up appears to offer a good indicator of the fragility of cognitive function. The list of 14 questions presented in PQ1 was presented again in a repeated procedure that was designated PQ2. The difference between these two measures (PQ1-PQ2) was designated D∆. These procedures for testing: 14-item test - graphia test - 14-item test were presented in an identical manner every second over 10 days (Days 1, 3, 5, 7, 9, 11, 13, 15, 17, and 19) at the same hour-of-day on test days in order to hold constant testing procedures over daily curriculum and any clinical interventions that the patients may be subject to.
The patients responded to questions before personal details and after to questions spatial orientation and after to questions regarding temporal orientation.
Statistical Analysis
The results consisting of PQ1 and PQ2 scores, min spent writing and D∆ (see above) was calculated as means and standard deviations of the AD patient group and the healthy control group over consecutive days of testing. Student’s t-tests were used to test for pairwise differences for each of the parameters. Pearson’s correlation coefficient was used to assess the relationship between writing-time and PQ1 and writing-time and D∆ over patients and test days.
RESULTS
AD Patients: Cognitive Performance Over PQ1 and PQ2
The cognitive performance of the AD patients deteriorated from PQ1 to PQ2 (see Table 3) with t-values reaching significance levels over all 10 days of testing.
Correlation Coefficients: PQ1 Versus Writing-Time and Writing-Time Versus D∆
The correlation coefficients of both the PQ1 performance and writing-time relationship and the writing-time and D∆ relationship were all positive and highly significant (see Table 3).
There were highly significant, positive correlations between the 1st, PQ1, test and extent writing ability, graphia, and the latter with cognitive performance deterioration, D∆, over test days summated over all 30 patients. Fig. (2) presents the correlation analysis (coefficient and slope) between PQ1 and writing-time (top) and writing-time and D∆ over all 10 days of testing.
Concurrently, there were highly significant, positive correlations between the 1st, PQ1, test and extent of writing ability, graphia, and the latter with cognitive performance deterioration, D∆, over 30 patients summated over all 10 test days. Fig. (3) presents the correlation analysis (coefficient and slope) between PQ1 and writing-time (top) and writing-time and D∆ over all 30 patients.
Correlation Analyses Between Functional Measures
Product moment correlations between the functional estimations of AD, ADL, IADL and MMSE, and the neuropsychological assessments of cognitive performance, PQ1, D∆ and PQ2, were carried out on the data obtained from the 30 AD patients. It was observed that (i) ADL, IADL and MMSE all correlated significantly with PQ1, albeit at different levels of significance, (ii) IADL and MMSE correlated significantly with D∆, (iii) ADL and MMSE correlated significantly with PQ2, and (iv) ADL and IADL correlated significantly with MMSE.
Comparison AD Patients with Healthy Controls
There was a marked impairment in the AD patients and the healthy controls for the expression of extent writing ability, graphia, and the extent of cognitive performance deterioration, D∆. Table 5 (A and B) presents the means±SD as well as t-values over all 10 days of testing.
The extent of dysgraphia by the AD patients was found to be quite advanced in comparison with the healthy controls (see Fig. 4).
Concurrently, the extent of cognitive performance deterioration, D∆, from the 1st to the 2nd test, was found to be markedly advanced in comparison with the healthy controls (see Fig. 5).
DISCUSSION
The present study examined the relationships between initial cognitive performance (PQ1), the deterioration in cognitive performance following a letter-writing task (PQ1-PQ2=D∆) and dysgraphia in a group of AD patients presenting a moderate to relatively severe stage of disorder, and comparisons with a matched group of healthy controls. The results may be summarized as follows:
Both the correlations between PQ1 and PQ2 over all the test days and the deterioration of performance from PQ1 to PQ2 over all test days were markedly significant, implying that the cognitive performances over the different measures were linkedstrongly. (ii) The relationships between initial cognitive performance (PQ1) and extent and dysgraphia over both patients and test days were markedly strong. (iii) The relationships between dysgraphia and cognitive deterioration (D∆) were also markedly strong. (iv) In comparison with the matched healthy controls, the AD patients demonstrated deficits in initial cognitive performance (PQ1), the extent to which dysgraphia was expressed and the extent of deterioration due to the insertion of the writing (graphia) test; the extent of deterioration due to the insertion of the writing (graphia) test extremely marked (see Fig. 5). (v) ADL, IADL and MMSE correlated significantly with PQ1, whereas IADL and MMSE correlated significantly with D∆, ADL and MMSE correlated significantly with PQ2, and ADL and IADL correlated significantly with MMSE (see Table 4); furthermore, writing-time performances correlated significantly with ADL, IADL, MMSE and D∆. In particular, the association between writing-time (or expression of dysgraphia) and D∆, the deterioration from the 1st cognitive test (PQ1) to the 2nd (PQ2), was markedly significant (R2 = 0.850).
Dysgraphia occurs during both the earlier as well as the later stages during the clinical course of AD [36-38] and is associated with attentional, motor and memory deficits that develop during disorder progression [39]. It has been suggested to be a more sensitive indication of language deficits in AD than anomia [22]. Yoon et al. [40] have observed that a sample of 35 patients presenting early onset AD, with a severe degree of hypometabolism in the parietal brain region, exhibited not only linguistic errors but also visuoconstructional manifestations (derived from Hangul scripts) of dysgraphia that were associated with cognitive impairments in multiple domains. In a sample of 75 AD patients and 20 healthy controls that were set Hangul writing tasks, it was found that the writing performance of the AD group was significantly defective with a profusion of different types of errors emerging with disorder progression [41]. PET imaging of glucose metabolism indicated that the hypometabolism in the right occipitotemporal lobe and left temperoparietal lobe was linked to Hangul writing impairment [41], in accordance with lesioning studies of dysgraphia [42]. In a sample of 52 Japanese patients presenting mild AD and 22 healthy controls, writing ability composed of Kana writing-to-dictation and copying Kanji or dictational Kanji, and regional cerebral blood flow using SPECT were studied [43]. They observed that while Kana writing-to-dictation and copying Kanji were preserved in these AD patients, writing to dictated Kanji words was impaired. The impaired writing of dictated Kanji words was associated with dysfunctional cortical activity predominantly in the left frontal, parietal and temporal brain regions [43] consistent with other Japanese dysgraphia studies [44-46]. The present observations of dysgraphia associated with deficits in semantic memory that were exacerbated acutely by the writing task appear to fit current notions pertaining to the progressive performance impairments of AD patients within language and cognition domains from a staging perspective [47, 48].
The possible relationships between dysgraphia and the motor functioning domain in AD has provided novel insights of the cognitive nature of disorder [21] through which mild to moderate stage AD patients (n=59) and healthy elderly controls were tested over an extensive assessment of both the central and peripheral components of writing; the former performed less effectively than controls over a broad spectrum of writing measures. Although a predominantly lexical disorder was observed, there were multiple indications of associated disorders located at different stages in the writing/spelling system (e.g. phonological route, graphemic buffer, allographic store, graphic motor patterns). The authors concluded that there exist heterogeneous profiles of dysgraphia with primary signs of writing impairment in AD originating from changes at different points in the brain networks that subservewriting and spelling performance [21]. In this regard, the possibility of related motor deficits in dysgraphia ought to be considered since there is evidence for altered parietal-motor connections in AD [49]. It has been found too that sensory-motor plasticity is impaired in the motor cortex (see above parietal cortex glucose hypometabolism) of AD at an early stage of the disease [50]. Furthermore, clear differences between AD patients and healthy control individuals have been found for visuomotor task measures demonstrating large effect size deficits by AD patients especially with visuomotor task progression through its varying conditions [51]. Sitek et al. [52] have observed dysgraphia, primarily dysexecutive agraphia, in patients with frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Patients presenting ‘dysexecutive’ agraphia show not only difficulties in maintaining the effort inherent to writing but appear to lack the ability to organize their thoughts for expression in the written text. They seem to be lacking in the complicated functions underlying writing that encompass narrative coherence, planning, selective attention, etc, that are disturbed in executive function impairments.
The relationship between MMSE and dysgraphia in AD patients has been established [53, 54], although there remains a paucity of studies examining the associations involving ADL and IADL with dysgraphia. In the present study the expressions of dysgraphia (writing-time) as well as the expressions of cognitive deficits were related strongly to the assessments of functional impairment (MMSE, ADL and IADL). The question arises as to whether the functional deficits expressed by ADL, IADL and MMSE may have contributed to the cognitive performance deficits shown by PQ1, D∆, PQ2 and writing-time. Choi et al. [55] observed that the expression of extrapyramidal signs in AD patients, non-recipient of neuroleptics (dopamine antagonists) was associated with more impaired basic ADL and instrumental ADL functioning and with more depression symptoms. Several have indicated that dysgraphia is related to severity of AD disorder [56-58]. Hughes et al. [36] have demonstrated the relationships between extent of MMSE impairment and patterns of dysgraphia in AD. MMSE deficits in AD are linked to several co-morbidity domains, including cardiovascular, ear, nose and throat, genitourinary, musculoskeletal/integument and neurologic, as well as severity of impairment of ADL and Cumulative Illness Rating Scale for Geriatrics [59]. Expressions of dysgraphia ought to be considered against this background. Finally, it has been observed that both ADL and IADL fail to reflect caregivers’ burden and patients’ behavioral symptoms, including affective domains, which need to be assessed in conjunction with cognitive analyses [60].
In conclusion, marked relationships between dysgraphia and several measures of cognitive performance in AD patients were observed concomitant with consistent deficits by this sample in comparison with a matched group of healthy control subjects. Several measures of loss of functional integrity, MMSE, ADL and IADL, were found to be associated with both dysgraphia and impairments in cognitive performance. These findings imply that assessment of dysgraphia ought to contribute an additional diagnostic assessment that provides evidence of compromised functioning in motor, cognitive and emotional domains [61]. The loss of functional integrity in these AD patients was serious; nevertheless, the manifest benefits arising from a motor interventional set-up, e.g. physical exercise programs, for these patients ought not to be rejected outright [47].
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Special thanks are due to the patients who participated and the UVA structures.
Sources of funding. UVA and University of Gothenburg, Erasmus Foundation.


