All published articles of this journal are available on ScienceDirect.
Did Oncotype DX® Recurrence Score Accurately Predict the Risk of Recurrence in Breast Cancer? A 10 Year Period Study in a Single Institution
Abstract
The 21-gene Recurrence Score (RS) assay (Oncotype DX®) predicts the risk of recurrence and benefit from chemotherapy in estrogen receptor (ER) positive, Her-2/neu negative, node negative and, more recently, limited node-positive (≤3) breast cancer. The 21-gene RS is divided into low, intermediate and high risk groups corresponding to a likelihood of recurrence within 10 years of initial diagnosis. Clinicians utilize 21-gene RS to guide treatment, particularly whether to add adjuvant chemotherapy to endocrine therapy. This study aimed to determine if 21-gene RS accurately predicts the rate of recurrence with respect to each category. A cohort of 236 patients was studied retrospectively and analyzed, based on correlation between histologic and immunohistochemical (IHC) findings versus 21-gene RS stratification in relation to clinical outcomes.
In the cohort examined, no deaths occurred in all the patients studied. Six patients had recurrence or metastatic disease. Of these six patients, only one had been stratified to the high risk group by 21-gene RS analysis, while four were stratified to the low risk group, and one to the intermediate risk group. 21-gene RS accurately predicted 97% of the low RS stratified patients to avoid receiving chemotherapy. However, addition of chemotherapy in the treatment regimen for node positive, Her-2/neu positive, high Ki-67, and PR negative tumors may be beneficial regardless of 21-gene RS. Our investigation found that there is a high concordance rate between 21-gene RS and IHC of ER, progesterone receptor (PR), and Her-2/neu.
INTRODUCTION
The 21-gene Recurrence Score (RS) assay (Oncotype DX®) breast cancer test is a commercially available multigene reverse transcription-polymerase chain reaction (RT-PCR) test that has been clinically validated to predict the likelihood of distant recurrence (assuming patient takes 5 years of tamoxifen) for early-stage breast cancer. This test is intended for women with early-stage (Stage I or II), node-negative (more recently included limited node positive), estrogen receptor (ER+) positive and Her-2/neu negative invasive breast cancer. It includes 16 cancer genes which includes ER, Progesterone receptor (PR), Her -2/neu, proliferation index, with proliferation being most heavily weighted in formula and 5 reference genes.
Recurrence Score (RS) based on a mathematical algorithm generated from the results of the profiling assay stratifies patients into low- (L) (RS<18), intermediate- (I) (RS 18-30), and high- (H) (RS 30-100) risk groups corresponding to a likelihood of distant (metastatic) recurrence within 10 years of the initial diagnosis. While patients with scores in the low risk range appear to derive no benefit from the addition of adjuvant chemotherapy, patients with scores in the high risk range do appear to derivesignificant benefit from the addition of chemotherapy to their adjuvant regimen. For patients with intermediate scores, the benefits of chemotherapy remain unproven. Thus 21-gene RS test has both a prognostic and predictive value, since it provides information about how likely (or unlikely) the breast cancer is to recur, and since it predicts the likelihood of benefit from chemotherapy in addition to endocrine therapy for those patients who have a low or high risk score. Commercially available since 2004, Oncotype DX® was arguably the first genomic biomarker assay to become available for breast cancer treatment decisions.
Clinicians utilize 21-gene RS to help facilitate adjuvant treatment decision making, particularly whether to add chemotherapy to endocrine therapy. In the past decade, multiple studies have shown that the utilization of 21-gene RS test in clinical practice is frequently responsible for changing adjuvant treatment decisions, with one meta-analysis demonstrating that 21-gene RS changed the clinical-pathological adjuvant chemotherapy treatment recommen-dation in 33.4% of patients [1]. 21-gene RS has been validated in a number of clinical studies, including the National Surgical Adjuvant Breast Program (NSABP) B-14 and B-20 trials [2, 3], in addition to a number of other studies that have investigated its prognostic utility [4-8].
The 21-gene RS assay is not without its costs, with the test currently retailing for about $4,000 per patient in the United States. Although this cost should be examined in the context of its clinical benefits, a question that arises is whether clinical, histologic, and immunohistochemical (IHC) tests alone without the gene assay have a similar prognostic and predictive value. Cuzick et al., did such a study and found that the IHC4 score provided similar if not better prognostic information as 21-gene RS assay [9]. In fact, many authors argue that there is no additional prognostic information from 21-gene RS when compared to routine and classical clinico-pathologic factors including age, nodal status, tumor size, grade, and ER, PR, Her-2/neu and Ki-67 are done [10, 11]. Overall, low levels of ER and PR expression, a high level of proliferation index by Ki-67, and Her-2/neu overexpression or amplification predict a higher risk of recurrence. Given that 21-gene RS predicts 10 year risk of recurrence, we investigated a total of 236 patients with breast cancer at our institution who had morphologic evaluation, IHC results for ER, PR, Her-2/neu and Ki-67 and 21-gene RS over 10 year period. Our investigation is to follow up on our patients population with known 21-gene RS to see if it truly predicted recurrence correctly.
MATERIALS AND METHODOLOGY
Patients
The Pathology computer database was searched retrospectively for patients who had invasive breast cancer and Oncotype DX® test results in our institution, after obtaining our institutional IRB approval. A total of 236 cases out of 1,953 patient breast cancer sample (11.5%) spanning a period of ten years (September 2003 to April 2013) were identified that fit these criteria. Oncotype DX® test is sent by clinician’s request in our institution. A few of the cases sent for Oncotype DX® test had Her-2/neu positive, T3 and/or ER negative tumors which are outside the guidelines for the indication for Oncotype DX® test. The clinical information and follow-up were obtained from the patients’ electronic medical record and our pathology database computer system PowerPath; data collected included all pathology breast tumor parameters (tumor size, tumor grade, modified Bloom and Richardson score, lymph node status, ER and PR expression, Her-2 by IHC and FISH tests, and Ki-67. All cases are reviewed by four expert breast pathologists in our institution. The clinical and management data that were collected included the date of the last documented patient visit, adjuvant chemotherapy and endocrine therapy. The clinical outcomes were then reviewed for each case and included recurrence, metastasis, or death as an endpoint.
Results
A total of 236 cases were identified spanning a period of ten years (2003 to 2013). Of these cases, two were male patients (2/236; 0.8%). Patients ranged from 24 to 79 years old at the time of diagnosis (mean age of 54 years). Over time we observed an increasing trend of breast cancer cases sent by clinicians for genetic analysis by Oncotype DX®, with a substantial increase in the number of sent tests from 2003 to 2011 and slight decline in 2012. (See Fig. 1)
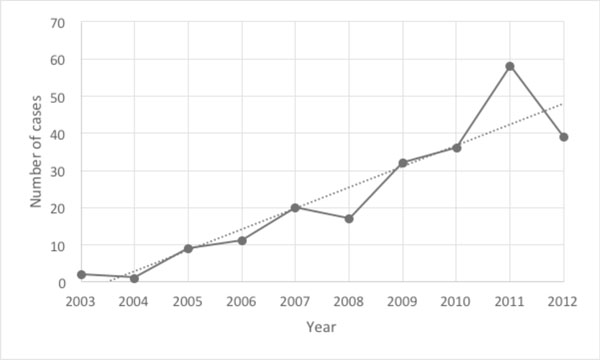
Chart showing number of Oncotype DX® tests sent by each year.
Tumor Characteristics and Histological/Immunohistochemical Findings
Of the total 236 patients, 38% were classified as Grade 1 tumors (90/236), 50% were classified as Grade 2 tumors (119/236), and 12% were classified as Grade 3 tumors (27/236). Of the total 236 cases, 96% were found to be T1 or T2 tumors (227/236) and 4% were found to be T3 tumors (9/236). No cases were found to be T4 tumors. Forty-seven cases were found to have one or more positive lymph nodes, but less than three, (47/236; 20%), with all of the remaining patients having no metastatic disease to the lymph nodes.
The IHC staining for ER, PR, and Her-2/neu status were as follows: 99% ER positive (234/236) and 1.0% ER negative (2/236), 95% PR positive (225/236) and 5% PR negative (11/236), 98% Her-2/neu negative and four positive for Her-2/neu (2%; 4/236) by both IHC and FISH respectively. The proliferation index was also determined for each case, and was considered high when Ki-67 was greater than or equal to 20%. Of the 236 total cases, 43 were found to have elevated Ki-67 (18%) and 169 were found not to be elevated (82%).
Oncotype DX® Risk Score (RS) Results
Of the 236 cases, 65% were stratified into the low-risk (LR) group by genetic analysis (152/236), 29% into the intermediate risk (IR) group (69/236), and 6% into the high risk (HR) group (15/236). The morphologic and IHC characteristics of each tumor were then examined with respect to stratification by the RS score (Table 1).
Data summary of all patients.
| Low Risk | Intermediate Risk | High Risk | All Cases | |
|---|---|---|---|---|
| Tumor Size (average) | 2.28 cm | 2.38 cm | 1.6 cm | 2.3 cm |
| T Score (average) | 1.56 | 1.52 | 1.2 | 1.57 |
| T1 | 74/152 (49%) | 35/69 (51%) | 12/15 (80%) | 121/236 (51%) |
| T2 | 71/152 (47%) | 32/69 (46%) | 3/15 (20%) | 106/236 (45%) |
| T3 | 7/152 (4%) | 2/69 (3%) | 0/15 (0%) | 9/236 (4%) |
| Nottingham Score (average) | 5.49 | 6.39 | 6.93 | 5.84 |
| Grade (average) | 1.59 | 1.97 | 2.13 | 1.74 |
| G1 | 74/152 (49%) | 14/69 (20%) | 2/15 (13%) | 90/236 (38%) |
| G2 | 67/152 (44%) | 43/69 (62%) | 9/15 (60%) | 119/236 (50%) |
| G3 | 11/152 (7%) | 12/69 (18%) | 4/15 (27%) | 27/236 (12%) |
| Positive Lymph Nodes | 36/152 (24%) | 11/69 (16%) | 0/15 (0%) | 47/236 (19%) |
| ER Positive | 151/152 (99%) | 69/69 (100%) | 14/15 (93%) | 234/236 (99%) |
| ER Negative | 1/152 (1%) | 0/69 (0.0%) | 1/15 (7%) | 2/236 (1%) |
| PR Positive | 149/152 (98%) | 65/69 (94%) | 11/15 (73%) | 225/236 (95%) |
| PR Negative | 3/152 (2%) | 4/69 (6%) | 4/15 (27%) | 11/236 (5%) |
| Ki67 Positive | 16/152 (11%) | 17/69 (25%) | 10/15 (67%) | 43/236 (18%) |
| Ki67 Negative | 136/152 (89%) | 52/69 (75%) | 5/15 (33%) | 193/236 (82%) |
| HER2 Positive | 0/152 (0%) | 0/69 (0.0%) | 4/15 (27%) | 4/236 (2%) |
| HER2 Negative | 152/152 (100%) | 69/69 (100%) | 11/15 (73%) | 232/236 (98%) |
Data showing RS and actual recurrence/metastasis.
| Low Risk | Intermediate Risk | High Risk | |
|---|---|---|---|
| Number (percentage) | 152 (65%) | 69 (29%) | 15 (6%) |
| Patients with recurrence/metastasis | 4 (3%) | 1 (1%) | 1 (7%) |
| Chemotherapy received | 8/152 (5.3%) | 27/69 (39.1%) | 11/15 (73.2%) |
Data of patients who had recurrence/metastasis.
| No. | Histologic Type |
RS | Grade | T Stage | Lymph Node Status |
ER | PR | HER2 | Ki67 | Recurrence | Metastasis | Chemo | Time Until Event (Month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IDC | Low | 1 | 1 | Negative | Positive | Positive | Negative | Low | Yes | Yes - widespread osseous |
No | 91 |
| 2 | IDC | Low | 1 | 2 | Positive | Positive | Positive | Negative | Low | Yes | No | No | 41 |
| 3 | IDC | Low | 2 | 1 | Negative | Positive | Positive | Negative | Low | No | Yes - to thoracic spine and liver |
Yes | 48 |
| 4 | ILC- pleomorphic |
Low | 3 | 1 | Positive | Positive | Positive | Negative | Low | No | Yes - abdominal carcinomatosis |
No | 18 |
| 5 | IDC | Intermediate | 2 | 2 | Negative | Positive | Negative | Negative | Low | No | Yes - to femoral head | Yes | 63 |
| 6 | IDC | High | 2 | 1 | Negative | Positive | Positive | Negative | High | No | Yes - to lymph node | Yes | 33 |
IDC: invasive ductal carcinoma, ILC: invasive lobular carcinoma.
Of the LR stratified patients, 49% were found to have grade 1 tumors (74/152), 44% were found to have grade 2 tumors (67/152), and 7% were found to have grade 3 tumors (11/152). In addition, 49% were found to have T1 tumors (74/152), 47% were found to have T2 tumors (71/152), and 4% were found to have T3 tumors (7/152) from the LR group. Thirty-six of the low risk stratified patients had one or more positive lymph nodes (36/152, 24%). With respect to IHC analysis, 1% were found to be ER negative (1/152), 2% of the tumors were found to be PR negative (3/152), and no cases were found to be Her-2/neu positive (0/152). Ki-67 was elevated in 11% of the LR patient tumors (16/152).
Of the IR stratified patients, 20% were found to have grade 1 tumors (14/69), 62% were found to have grade 2 tumors (43/69), and 18% were found to have grade 3 disease (12/69). In addition, of the IR stratified patients, 51% were found to have T1 tumors (35/69), 46% were found to have T2 tumors (32/69) and 3% were found to have T3 tumors (2/69). The majority of the IR patients were found to have negative lymph nodes (N0; 58/69, 84%). None of the IR stratified cases were found to be ER negative, 6% (4/69) were PR negative and no cases were Her-2/neu positive. The Ki-67 was elevated in 25% of IR patient tumors (17/69).
Of the HR stratified patients, 13% were found to have grade 1 tumors (2/15), 60% were found to have grade 2 tumors (9/15), and 27% were found to have grade 3 disease (4/15). In addition, of the HR stratified patients, 80% were found to have T1 tumors (12/15) and 20% were found to have T2 tumors (3/15), while all of the HR patients were found to have negative lymph nodes (N0; 15/15). Of the HR stratified tumors, 7% (1/15) were ER negative, 27% (4/15) were PR negative and 27% were Her-2/neu positive (4/15). The Ki-67 was elevated in 67% of the HR patient tumors (10/15).
Eight patients (5.3%) from the low 21-gene RS received adjuvant chemotherapy and 26.7% of patients who had the high 21-gene RS did not receive adjuvant chemotherapy. The reasons were due to individualized preferences and co-morbidity issues.
Oncotype DX® currently also report ER, PR and Her-2/neu status by RT-PCR method. The discordance rate between IHC from our institution and RT-PCR by Oncotype
DX® for ER, PR and Her-2/neu status was found to be low: 1% (2/236), 9% (21/236) and 0% (0/236), respectively.
Adjuvant Therapy and Clinical Course
The average and median clinical follow-up time for all of the cases included in the study were 28.8 months and 23 months respectively, ranging from 1 month to 125 months. We found that of the HR, IR and LR stratified patients, 11/15 (73.2%), 27/69 (39.1%) and 8/152 (5.3%) received adjuvant chemotherapy, respectively. (Table 2) All patients received adjuvant endocrine therapy including the six patients who had recurrence. No deaths occurred in any of the patients studied. Six of the total patient cohort had a significant event, including recurrence or metastasis (Table 3). The average time of onset of the significant event was 45 months. Of the six cases, four were stratified into the low risk group, one into the intermediate group and one into the high risk group by 21-gene RS analysis. All of these cases were ER positive, one was PR negative (1/6; 16.7%), all were HER-2/neu negative and one showed elevated Ki-67 (1/6; 16.7%). Two of these cases had positive lymph node status (2/6; 33.3%). Only three of these patients had received chemotherapy, one from each risk group, respectively.
DISCUSSION
With the advancement of genetic analysis and the development of genetic profiling assays, particularly Oncotype DX®, there has been a paradigm shift in what oncologists base their adjuvant chemotherapy management upon with a move away from clinico-pathologic criteria alone such as tumor size, lymph node status, tumor grade and IHC results. Indeed, recent investigations show that medical oncologists change management in about 26 to 44% of cases based on genetic analysis findings through 21-gene RS [12-16].
Our study shows that there was a substantial increase in the number of breast cancer cases that were sent for Oncotype DX® testing by the oncologists’ request at our institution (from 3 cases total in late 2003 and 2004 to an average of 42 cases during the years 2010 to 2012). Overall, the average rate of Oncotype DX® request by clinician is approximately 16% in our institution. When Oncotype DX® was utilized; patients stratified in the high risk group with a high RS were much more likely to receive adjuvant chemotherapy as compared to those patients stratified in the low RS or the intermediate RS group. However, the patients in the high risk stratified group also tended to have more concerning clinico-pathologic and IHC findings as well, including having positive Her-2/neu status, elevated Ki-67, and negative PR status within ER-positive breast cancers.
A number of recent studies have investigated the relationship between the impact of cost and decision making based on 21-gene RS results as compared to histologic and IHC evaluations. One study by Biroschak et al., examined 50 patients with ER-positive, lymph node negative breast cancer analyzed by 21-gene RS [12]. The investigators found that all cases in their study group that were categorized as high risk by the RS were categorized as high risk based on the pathologist’s assessment as well, and that the vast majority of the cases that were categorized as low or intermediate risk by RS were also categorized as low or intermediate risk by the pathologist (96%). In a study by Zbytek et al., the investigators found that mitotic score based markers of proliferation index including Ki-67, phosphohistone H3, and the hematoxylin and eosin (H&E) mitotic score defined by the Nottingham grading system were useful in assessing tumor grade, proliferation and prognosis, and that proliferation markers correlate with the recurrence score [17]. In addition, a study by Allison et al., investigated whether routinely available pathologic parameters including tumor size, histologic type, Nottingham grade and lymphatic invasion could predict the 21-gene RS. They found that grade 1, high PR positivity, low Ki-67 breast cancers correlated well with low RS stratified patients and grade 3, low PR, high Ki-67 breast cancers correlated well with high RS stratified patients [11]. Additional studies on specific pathologic features have been shown to correlate with the 21-gene RS, including histologic features, mitotic count, and hormonal receptor expression [12, 18-21]. In a 2011 study by Cuzick et al., the authors showed that all four of the IHC markers, ER, PR, Ki-67, and HER2 provided independent prognostic information in the presence of classical clinico-pathologic factors including age, nodal status, tumor size, grade and endocrine treatment [9]. Their study suggested that the amount of prognostic information contained in these IHC4 assays is similar to that in the Genomic Health RS in early breast cancer. Additionally, a recent study by Klein et al. demonstrated that an equation (the Magee equation) could reliably predict the RS generated by genetic analysis through Oncotype Dx® (when the intermediate category was eliminated) by using standard morphologic and IHC variables [10]. However, another study that investigated 465 patients with ER-positive breast cancer and 0 to 3 positive lymph nodes showed that the 21-gene RS was more accurate at predicting recurrence rate than clinico-pathologic features alone [22]. A study by Tang et al. found that 21-gene RS and clinico-pathologic information from Adjuvant!Online provided independent prognostic contributions in node negative, ER positive breast cancer patients. However, only 21-gene RS consistently predicted chemotherapy benefit across various endpoints [23]. Results from this study which composed of larger parent B-20 cohort showed that Adjuvant!Online predicted chemotherapy benefit, but only in overall survival, and the magnitude of prediction was less than that of the 21-gene RS.
Our investigation is the first 10 year period clinical follow-up study of 21-gene RS in a cohort of 236 patients in a single institution to see whether 21-gene RS accurately predicted the rate of recurrence with respect to each risk category and the association of histologic categorization. Notably, recurrence and metastatic events occurred in six of the patients (2.5%) in our cohort. Among the six patients, only one of these patients was stratified in the high RS group, while one had been stratified in the intermediate RS group, and four in the low RS group, with a mean follow-up of 49 months (4.1 years). Three patients with low RS who had significant events had small tumor (T1-T2), ER and PR positive, low Ki-67 and Her-2/neu negative breast cancers but two of three patients had sentinel lymph node involvement. One patient who had a low RS had grade 3 invasive lobular carcinoma and she received chemotherapy despite the low RS and recurred with carcinomatosis in abdomen after 18 months. Our finding shows that 21-gene RS accurately predicted 97% of the low RS stratified patients.
The cost-saving and avoiding side-effects from the adjuvant chemotherapy would be significantly beneficial from having 21-gene RS test. However, overall, morphologic and IHC findings can predict 21-gene RS. Patients who were stratified in the high risk group tended to have tumors with higher Ki-67 (67% in the high risk group versus 11% in the low risk group). High RS group patients also had a higher percentage of PR negative tumors (27% PR negative in the high risk group versus 2% in the low risk group).
Our investigation found that there is a high concordance rate between Genomic Health RT-PCR and IHC evaluation for ER, PR, and Her-2/neu. Of the cases examined, the ER status discordance rate was only 1%, PR status discordance rate was 9%, and there was no discordance between IHC evaluation and RT-PCR analysis with 21-gene RS in the determination of Her-2/neu. Thus, the discordance rates for the determination of tumor hormone status were low between evaluation with IHC and evaluation by Genomic Health RT-PCR. Prior studies have also supported this notion, with a 2012 study by Kraus et al. demonstrating high concordance between IHC and Genomic Health RT-PCR for ER and PR (98.9% and 94.2%, respectively) with IHC being slightly more sensitive [24]. A recent study by Park et al., demonstrating high concordance between ER and PR by IHC with Genomic Health RT-PCR for ER and PR (98.9% and 91.3% concordance rates, respectively), although their data showed poor positive percent agreement for HER2 by fluorescent in situ hybridization (FISH) (positive percent agreement for HER2 was 0% (0/2 cases with positive HER2) while negative percent agreement was 100% (245/245 cases with negative HER2)) [25]. A 2011 study by Dabbs et al., investigated the concordance rate between IHC/FISH and Genomic Health RT-PCR HER 2 assay, and they found an unacceptable false negative rate for HER2 status with the Genomic Health assay results [26].
Our weaknesses of the study are as follows: 1. although our clinical follow-up includes 10 year period, the actual average/mean follow-up time is short which could explain why the rate of recurrence/metastasis is low, and 2. our cohort had small patient samples. Further study with a longer clinical follow-up and larger cohort study is needed.
In summary, patients who had low RS by 21-gene RS test with node positive, high Ki-67, high tumor grade and PR negative tumors may need to consider for adding adjuvant chemotherapy. Further study is needed to be done to confirm benefit of adjuvant chemotherapy on patients who had low RS by 21-gene with high Ki-67, high tumor grade, and PR negative tumors. Also, there is a high concordance rate between Genomic Health RT-PCR and IHC evaluation for ER, PR, and Her-2/neu in our institution.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We thank our oncologist Sara Hurvitz MD, who provided invaluable insight to our manuscript.


