All published articles of this journal are available on ScienceDirect.
Analysis of the Pathogenesis of Secondary Airflow Limitation in Tuberculosis based on ILC3 Chemotaxis
Abstract
Introduction
Tuberculosis-associated obstructive pulmonary disease (TOPD) remains poorly understood; accumulating evidence implicates innate lymphoid cells (ILCs), especially group 3 (ILC3), in post-tuberculous airway remodelling. We aimed to clarify whether CXCL13-CXCR5–directed ILC3 chemotaxis contributes to secondary airflow limitation.
Methods
In a case-control study, 34 patients with TOPD, 34 with stable chronic obstructive pulmonary disease (COPD) unrelated to tuberculosis, and 34 healthy controls were recruited. Peripheral blood and broncho-alveolar lavage fluid (BALF) were collected within 24 h of enrolment. Total ILCs and subsets were quantified using multiparameter flow cytometry; ELISA was used to measure CXCL13, CXCR5, IFN-γ, IL-23, IL-17, and IL-22. Group differences were analysed with one-way ANOVA or the Friedman test (p < 0.05).
Results
Circulating ILC3 frequency was reduced in TOPD versus COPD and controls (p < 0.001). Conversely, the BALF ILC3 proportion was markedly higher in TOPD than in COPD and control subjects (p < 0.001). TOPD patients exhibited the greatest systemic elevation of CXCL13, IL-23, IL-17, and IL-22 (all p < 0.01) and the highest BALF levels of CXCL13, CXCR5, and the same cytokines (all p < 0.001). IFN-γ levels and BALF ILC1 counts were also elevated, whereas changes in ILC2 were restricted to peripheral blood.
Discussion
The reciprocal pattern of diminished peripheral yet enriched pulmonary ILC3, together with a CXCL13-CXCR5 gradient, supports the selective recruitment of ILC3 from blood to lung parenchyma following Mycobacterium tuberculosis infection, sustaining type 3 inflammation beyond microbiological cure. Although the cross-sectional design and modest sample size limit causal inference, the findings align with experimental models and extend knowledge to human TOPD.
Conclusion
TOPD is characterized by CXCL13-CXCR5–mediated trafficking of ILC3 that amplifies IL-23/IL-17/IL-22–driven airway inflammation, indicating this chemokine axis as a promising biomarker and therapeutic target for post-tuberculous airflow obstruction and progressive pulmonary decline.
1. INTRODUCTION
Previous reports have identified several factors that predict the severity of COPD among patients with tuberculosis, including smear-positive disease, extensive lung involvement before antituberculosis treatment, reduced imaging improvement after treatment, and delayed initiation of tuberculosis treatment [1]. A history of previous tuberculosis is strongly associated with the severity of COPD [2]. Our previous study found that the prevalence of airflow limitation secondary to TB in southern Xinjiang was approximately 21.8% [3], and this population had more severe clinical symptoms and poorer lung function.
Current understanding suggests that adaptive immune mechanisms mediated by CD4+ and CD8+ T lymphocytes constitute the principal pathway for Mycobacterium tuberculosis eradication [4]. However, in recent years, through deeper study of innate immunity research, it has been found that innate lymphoid cells play a protective role in the early immune process of tuberculosis [5]. At the same time, innate immunity is also involved in the pathogenesis of COPD [6]. Recent studies have shown that the CXCR5/CXCL13 axis is closely linked to the control of Mycobacterium tuberculosis (M. tb). Its specific mechanism of action may be related to the fact that infection upregulates the expression of CXCR5 on peripheral blood innate lymphoid cell 3s (ILC3s) and increases the level of its ligand, CXCL13, in human plasma [5]. These collective findings suggest that host-pathogen immunological interactions within the pulmonary microenvironment may significantly influence the development of TB-associated obstructive pulmonary disease. The present study aims to investigate the role of innate immunity in airflow limitation secondary to TB infection, focusing on the chemotaxis of the CXCL13-CXCR5 axis within the ILC subpopulation, and to provide a new research direction for the pathogenesis of TOPD.
2. MATERIALS AND METHODS
2.1. Selection and Grouping of Study Subjects
The study cases were obtained from patients with TOPD, patients with stable COPD and normal subjects who were diagnosed at the outpatient or inpatient departments of TB and respiratory departments of the Fourth Clinical Medical College of Xinjiang Medical University, the Second People's Hospital of Kashgar, the People's Hospital of Shule County and the People's Hospital of Yecheng County from March 2022 to January 2024 and who fulfilled the enrolment criteria, with 34 cases in each group. COPD patients were further divided into two different groups based on their history of TB infection: the TB-associated obstructive pulmonary disease group and the COPD group. Clinical data, including gender, age, height, and weight, were collected from the three groups. The study was designed as a case-control investigation, with healthy subjects serving as the control group and patients with COPD or TOPD comprising the case groups.
2.2. Diagnostic Inclusion and Exclusion Criteria
All patients met GOLD criteria for COPD diagnosis. Inclusion criteria were irreversible obstructive dysfunction (FEV1< 80% predicted, FEV1/FVC< 70% post-bronchodilator), absence of acute exacerbation, and age 18-75. Exclusion criteria included severe respiratory failure, pulmonary encephalopathy, mechanical ventilation, severe chronic respiratory diseases (e.g., lung cancer, pulmonary fibrosis), COPD-related pulmonary tuberculosis with smoking history, malignant tumors, chronic infectious/autoim-mune diseases, renal/cardiac diseases, diabetes, Alzheimer's, psychiatric conditions, HIV/immune deficiencies, and unwillingness to participate.
Diagnostic criteria for COPD secondary to tuberculosis [7]: previous history of tuberculosis, no history of smoking, and fulfillment of the diagnostic criteria for airflow limitation in COPD.
This clinical study was reviewed and approved by the Ethics Committee of the Fourth Clinical Medical College of Xinjiang Medical University (approval number:2020XE0131, Date: September 12, 2020], and written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
2.3. Specimen Collection and Processing
First, 5 ml of sodium heparin-anticoagulated whole blood samples were collected from all subjects during morning fasting. Whole blood designated for flow cytometry analysis was then immediately placed in flow tubes for antibody staining, while blood samples for serum collection were left at room temperature for 30 minutes and then centrifuged at 3000 rpm for 10 minutes. Extracted serum was immediately stored at -80°C. All patients were enrolled within 24 hours of enrolment.
All patients underwent bronchoscopy within 24 h of enrolment, and 5 mL of bronchoalveolar lavage fluid was collected, filtered through a single layer of gauze, centrifuged (centrifugation parameters: 3000 rpm for 10 min), and the supernatant was stored at -80°C in a refrigerator for examination.
2.4. Detection of ILC and its Subpopulations in Human Peripheral Blood by Flow Cytometry
100uL of anticoagulated whole blood was added to each sample tube, and the corresponding volume of Lin (CD3, CD4, CD8, CD14, CD15, CD16, CD19, CD20, CD33, CD34, CD203c, FcεRIα), along with antibodies against CD45, CD127, CRTH2, CD117 (BD, USA), was added to the sample tube. The red blood cell lysis buffer was incubated for 10 minutes, followed by centrifugation at 1,500 × g for 5 minutes. The supernatant was discarded, and the cells were washed once with phosphate-buffered saline (PBS). Subsequently, the cells were resuspended in PBS. Lymphocytes were gated by flow cytometry (FCM) (Fig. 1A), and doublets were excluded (Fig. 1B). The total innate lymphoid cells (ILCs) (CD45+ Lin- CD127+) were detected by FCM (Fig. 1C); ILC1 phenotype: CD45+Lin-CD127+CD117- CRTH2-; ILC2 phenotype: CD45+Lin-CD127+CD117+/-CRTH2+; ILC3 phenotype: CD45+Lin-CD127+CD117+CRTH2- (Fig. 1D: CONTROL, 1E: COPD, 1F: TOPD) and analysed using Kaluza software.
2.5. Detection of ILC and its Subpopulations in Human BALF by Flow Cytometry
Each sample tube was aliquoted with 2 mL of bronchoalveolar lavage fluid (BALF). Centrifugation was performed at 1,500 × g for 5 minutes, followed by the removal of the supernatant. The cell pellet was resuspended in a cocktail of lineage (Lin) antibodies (CD3, CD4, CD8, CD14, CD15, CD16, CD19, CD20, CD33, CD34, CD203c, FcεRIα; BD Biosciences, USA) along with CD45, CD127, CRTH2, and CD117 antibodies. Incubation was carried out for 10 minutes at room temperature under light-protected conditions. Centrifugation was repeated at 1,500 × g for 5 minutes, followed by aspiration of the supernatant. Cells were washed once with phosphate-buffered saline (PBS). Cells were resuspended in PBS, and Lymphocyte populations were gated as shown in Fig. (2A). The total innate lymphoid cells (ILCs) (CD45+ Lin- CD127+) were detected by FCM (Fig. 2B); ILC1 phenotype: CD45+Lin-CD127+CD117- CRTH2-; ILC2 phenotype: CD45+Lin-CD127+CD117+/-CRTH2+; ILC3 phenotype: CD45+Lin-CD127+CD117+CRTH2- (Fig. 2C: CONTROL, 2D: COPD, 2E: TOPD) and analysed using Kaluza software.
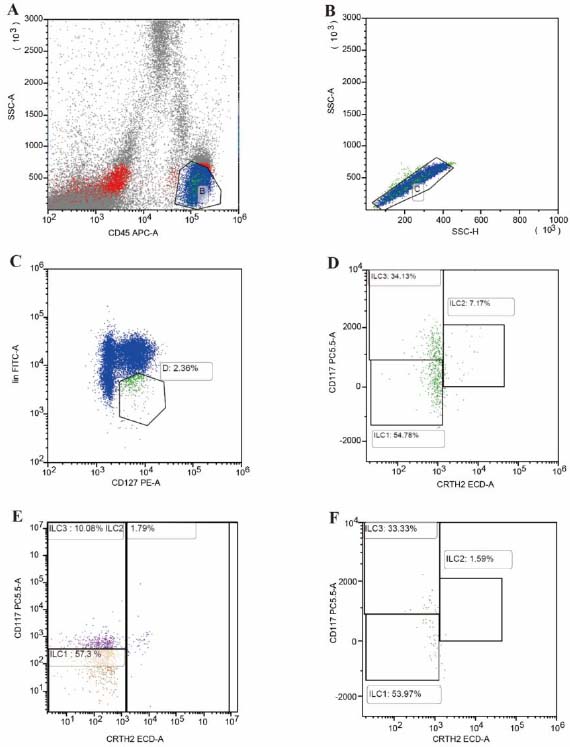
Proportion and subgroups of ILC in the peripheral blood of TOPD, COPD, and control groups. (A) Forward/side-scatter gating of lymphocytes. (B) Singlet discrimination. (C) Identification of total ILCs (CD45+ Lin− CD127+). (D–F) Representative dot-plots illustrating ILC1 (CD117− CRTH2−), ILC2 (CRTH2+), and ILC3 (CD117+ CRTH2−) subsets in Control (D), COPD (E), and TOPD (F) groups, respectively; percentages refer to frequency within lymphocytes.
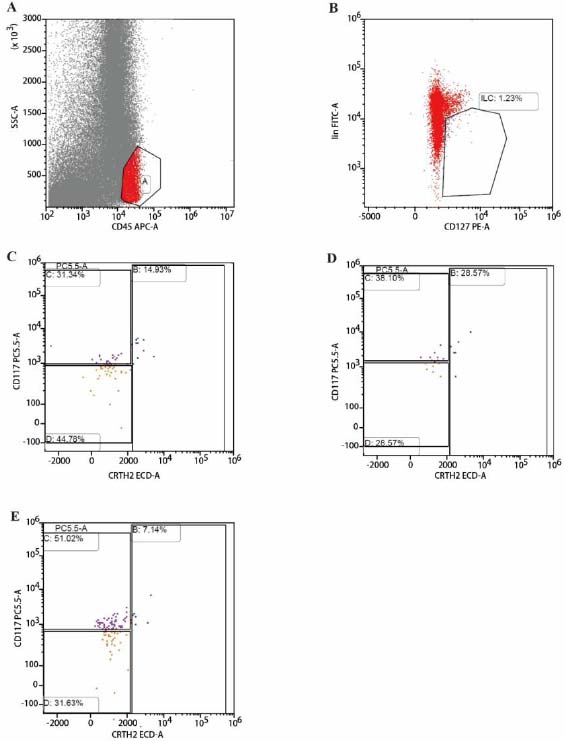
Proportion and subgroups of ILC in BALF in TOPD, COPD and control groups. (A) Representative FSC/SSC and CD45+ gating strategy defining lymphocytes. (B) Total ILCs identified as CD45+Lin−CD127+ events. (C) Control, (D) COPD, and (E) TOPD BALF showing ILC1 (CD45+Lin−CD127+CD117−CRTH2−), ILC2 (CD45+Lin−CD127+CD117±CRTH2+), and ILC3 (CD45+Lin−CD127+CD117+CRTH2−) subsets.
2.6. Enzyme-linked Immunosorbent Assay of Serum and BALF Samples
The concentrations of cytokines in peripheral blood serum were measured using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit, purchased from Wuhan Eliot Biotechnology Co. Briefly, the ELISA plate is coated with a capture antibody. Samples were added, followed by horseradish peroxidase-conjugated secondary monoclonal antibody. After washing three times with PBST, tetramethylbenzidine was added in the dark to develop the color. Absorbance was then measured using a spectrophotometer (Thermo, Waltham, MA, USA) at 450 nm and a reference wavelength of 570 nm. Concentrations were calculated from the standard curve.
2.7. Statistical Analysis
Statistical analyses were performed using SPSS 25.0 software (IBM Corporation, NY, USA), and graphs were plotted using Prism Version 8.00 (GraphPad Software, CA, USA) and EXCEL. The sample size of 34 per group was determined by power analysis, assuming a moderate effect size (Cohen’s f ≈ 0.25) with α = 0.05 and 80% power, to ensure sufficient statistical power.
When comparing the differences among the three groups of data, if the data were normally distributed, they were expressed by one-way ANOVA (‾χ±S); A Bonferroni correction was applied to adjust for multiple comparisons and control the overall type I error rate; if the data were skewed, they were expressed as median and quartile M(Q1 to Q3) using the Friedman test for multiple correlated samples, and the differences were considered to be statistically significant at P<0.05.
3. RESULTS
3.1. Baseline Data of Patients
There was no statistically significant difference (P > .05) in the comparison between the TOPD, COPD, and control groups in terms of age, gender, and BMI (P = 0.155) Table 1.
3.2. Changes in Peripheral Blood ILC Cells and their Subpopulations in the Three Groups
Total innate lymphoid cell (ILC) counts in peripheral blood were significantly reduced in the TOPD group compared to both controls (Cohen’s d = 1.604, P < 0.001) and COPD subjects (Cohen’s d = 0.607, P = 0.018), with no significant difference observed between controls and COPD patients. Analysis of ILC subsets revealed distinct patterns: ILC1 frequencies were markedly lower in COPD patients than in controls (Cohen’s d = 1.301, P < 0.001) and TOPD individuals (Cohen’s d = -1.646, P < 0.001). Conversely, ILC2 proportions were elevated in TOPD compared to controls (Cohen’s d = 1.273, P < 0.001) and COPD patients (Cohen’s d = 1.168, P < 0.001). Notably, ILC3 populations demonstrated significant reductions in TOPD relative to both controls (Cohen’s d = 0.680, P = 0.010) and COPD subjects (Cohen’s d = 2.462, P < 0.001) (Fig. 3).
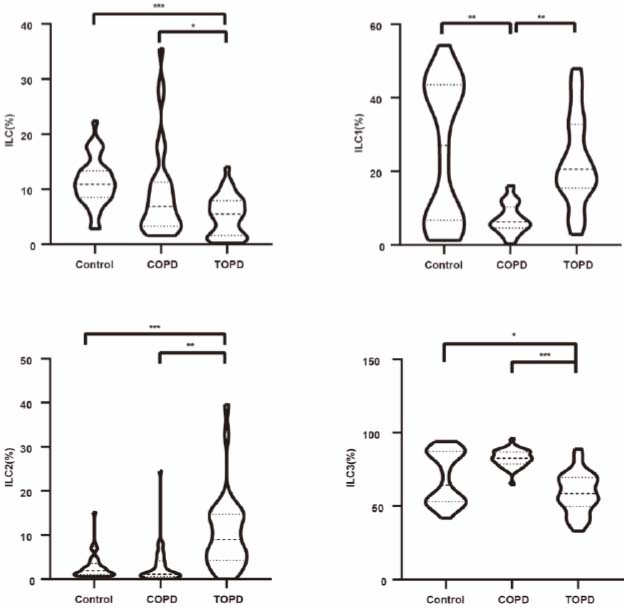
Proportion of ILC and subgroups in peripheral blood of TOPD, COPD, and control groups. *p<0.05, **p<0.01, ***p<0.001.
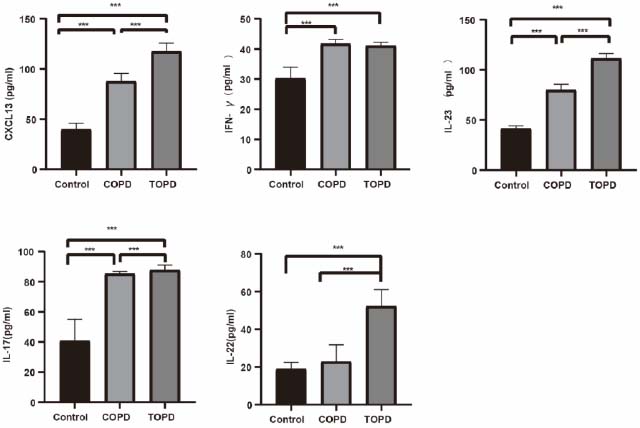
Comparison of peripheral blood levels of CXCL13, IFN-γ, IL-23, IL-22, and IL-17 inflammatory factors in the TOPD group, COPD group, and control group. *p<0.05, **p<0.01, ***p<0.001.
3.3. Peripheral Blood Cytokine Expression in the Three Groups
TOPD patients exhibited elevated CXCL13 levels compared to controls (Cohen’s d = 3.628, P < 0.001) and COPD subjects (Cohen’s d = 10.752, P < 0.001), with COPD also showing higher CXCL13 than controls (Cohen’s d = 6.720, P < 0.001). IFN-γ concentrations were reduced in both TOPD (Cohen’s d = -3.969, P < 0.001) and COPD groups (Cohen’s d = -4.119, P < 0.001) versus controls. IL-23 expression was significantly upregulated in TOPD compared to controls (Cohen’s d = 4.586, P < 0.001) and COPD patients (Cohen’s d = 18.360, P < 0.001). A stepwise increase in IL-17 levels was observed from controls to COPD (Cohen’s d = -4.345, P < 0.001) and further to TOPD (vs. controls: Cohen’s d = -4.051, P < 0.001; vs. COPD: Cohen’s d = -0.962, P = 0.007). IL-22 concentrations were significantly higher in TOPD than in both comparison groups (vs. COPD: Cohen’s d = -4.131, P < 0.001; vs. controls: Cohen’s d = -5.722, P < 0.001) (Fig. 4).
3.4. Changes of ILC Cells and their Subpopulations in BALF of the Three Groups
In bronchoalveolar lavage fluid, total ILC counts and ILC2 subsets showed no intergroup differences. However, ILC1 frequencies were reduced in TOPD compared to controls (Cohen’s d = 1.039, P = 0.006), while ILC3 populations were significantly expanded in TOPD relative to both controls (Cohen’s d = 1.799, P < 0.001) and COPD subjects (Cohen’s d = 1.263, P = 0.001). No significant differences were observed between COPD patients and controls in BALF ILC subsets (Fig. 5).
3.5. Cytokine Expression in BALF of the Three Groups
CXCL13 levels in BALF were markedly elevated in TOPD versus controls (Cohen’s d = 6.040, P < 0.001) and COPD patients (Cohen’s d = 5.979, P < 0.001). CXCR5 expression was diminished in TOPD compared to both groups (vs. controls: Cohen’s d = -15.787, P < 0.001; vs. COPD: Cohen’s d = -16.525, P < 0.001). IFN-γ concentrations were lower in controls than in COPD (Cohen’s d = 13.061, P < 0.001) and TOPD subjects (Cohen’s d = 15.706, P < 0.001). IL-23 demonstrated progressive increases from controls to COPD (Cohen’s d = -1.615, P < 0.001) and further to TOPD (vs. controls: Cohen’s d = -3.257, P < 0.001; vs. COPD: Cohen’s d = -2.415, P < 0.001). Conversely, IL-17 levels were reduced in TOPD compared to controls (Cohen’s d = -7.979, P < 0.001) and COPD patients (Cohen’s d = -4.349, P < 0.001), while IL-22 exhibited dramatic elevation in TOPD versus both comparison groups (vs. controls: Cohen’s d = 23.837, P < 0.001; vs. COPD: Cohen’s d = 14.956, P < 0.001) (Fig. 6).
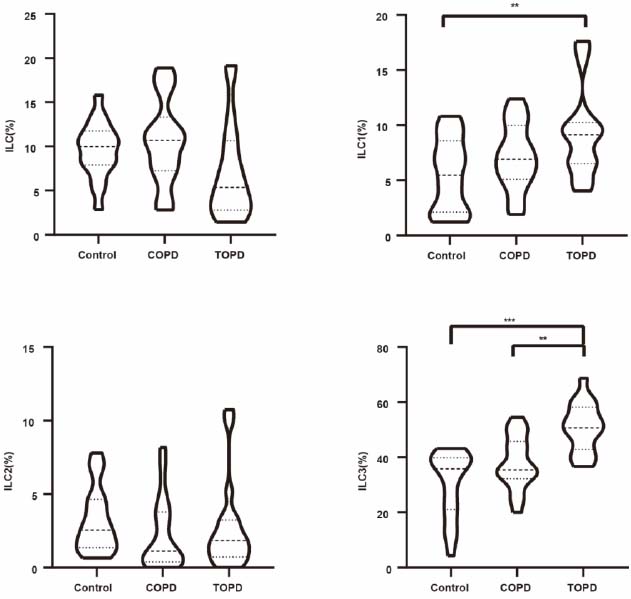
Proportion of ILCs and subgroups in BALF in TOPD, COPD, and control groups. *p<0.05, **p<0.01, ***p<0.001.
4. DISCUSSION
TOPD is characterized by persistent airflow limitation that occurs during or after intensive antituberculosis treatment in TB patients [7, 8]. A history of previous tuberculosis is strongly associated with the severity of COPD [2]. Studies on the pathogenesis of TOPD have not yet been fully clarified, and the present study aims to investigate the pathogenesis of TOPD from the perspective of changes in the immune environment of the lungs following TB infection.
Several studies [9-11] have pointed out that ILC is an important cell type involved in innate immunity during the early stage of TB infection, among which: the central role of ILC1s is to clear intracellular bacteria and participate in chronic inflammatory responses in the intestine [12]; ILC2s mainly have the roles of anti-parasitic infections, triggering airway hyper-responsiveness, and controlling lipid metabolism [13-15], and the main function of ILC3s is to play a mucosal protection against extracellular bacteria and fungi [16, 17].
Tuberculosis is a long-term, chronic inflammatory process, and prolonged stimulation leads to the high expression of ILC3 and its related inflammatory factors, including CXCL13, IL-23, IL-17, and IL-22, resulting in inflammatory responses in vivo. CXCL13 is an important chemokine in the body, which acts to chemotaxis leukocytes for directional movement, and some studies [18] have shown that CXCL13 only plays a chemotaxis role for C-X-C motif chemokine receptor 5 (CXCR5], which acts as a chemotactic agent, in which the response of CXCR5 to CXCL13 is specific. The CXCL13/CXCR5 axis is involved in the functional recruitment of lung ILC3s after M.tb infection, as well as in the localization of ILC3s to iBALT-associated granuloma [5]. High expression of CXCL13 in tuberculosis patients results in the recruitment of ILC3s to the lungs, where they exhibit high CXCR5 expression, facilitating the killing of Mycobacterium tuberculosis and contributing to early protective immunity. Recently, Shibali Das et al. utilized a mouse model to demonstrate that CXCR5 signaling plays a crucial role in the peripheral recruitment of ILC3 to the lungs during Mycobacterium tuberculosis infection [19].
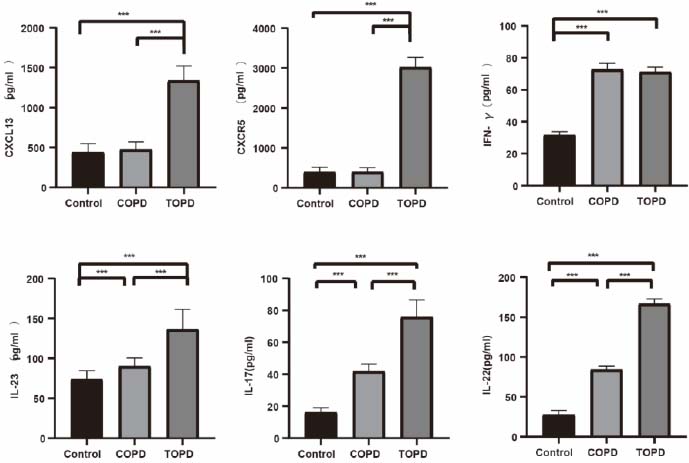
Comparison of the levels of CXCL13, CXCR5, IFN-γ, IL-23, IL-22, and IL-17 inflammatory factors in the BALF of the TOPD group, COPD group, and control group. *p<0.05, **p<0.01, ***p<0.001.
The CXCL13-CXCR5 axis is associated with the formation of iBALT [20]. Proinflammatory IL-17 can mediate inducible bronchial-associated lymphoid tissue (iBALT) generation [21], and iBALT is one of the hallmarks of the progression of chronic inflammation in COPD. Its number is directly proportional to the severity of lung function in patients with COPD [22]. In patients with COPD, IL-22 gene expression and protein levels were higher in COPD patients than in the healthy non-smoking group, and IL-22 promoted cigarette smoke-induced airway remodeling and lung function impairment [23]. In this study, CXCL13 was found to be significantly elevated in both peripheral blood and BALF in TOPD, and CXCR5 was also significantly elevated in BALF of TOPD patients, which was significantly different from that of COPD; however, there was no difference between CXCL13 and CXCR5 in BALF of patients with a stable stage of COPD, and at the same time, ILC3 was significantly elevated in the BALF of TOPD patients. Also, TOPD CXCL13, CXCR5, IL-23, IL-22, and IL-17 were highly expressed in the bronchoalveolar lavage fluid of the patients, suggesting that TB activates the CXCL13-CXCR5 axis. Thus, we speculate that TOPD may be related to the convergence of the CXCL13-CXCR5 axis, leading to the recruitment of peripheral blood ILC3 to the lungs for a sustained inflammatory response. However, whether IL-17 secreted by NCR-ILC3 and or IL-22 generated by NCR+ILC3 predominates in TOPD occurrence needs to be further investigated in depth.
The potential clinical significance of the CXCL13-CXCR5 axis in TOPD warrants further discussion. This chemokine axis may serve as a therapeutic target or a biomarker for disease severity. The neutralization of CXCL13 has been shown to reduce the formation of ectopic lymphoid structures and inflammation, a finding validated in preclinical autoimmune disease models, suggesting that targeting this pathway could modulate immune-driven pathological processes [24]. Furthermore, CXCL13 itself has been identified as a prognostic biomarker in chronic lung diseases, such as idiopathic pulmonary fibrosis, suggesting that elevated CXCL13 levels may reflect disease activity or severity in TOPD [25]. However, the specific causal role of the CXCL13-CXCR5 axis in the recruitment of ILC3s and the pathogenesis of TOPD requires further functional studies for validation.
It has been found that ILC1s also produce IFN-γ to limit Mycobacterium tuberculosis production in mice treated with IL-12 and IL-18, infected with TB [26]. It has also been noted that in TB-infected mice, lung IL-18Rα+ ILCs can differentiate into an ILC1-like subpopulation through the IFN-γ/stat-1 signaling pathway, and that this ILC1-like population reduces the bacterial population in TB-infected mice. The ILC1 subpopulation is elevated in COPD patients and correlates with the severity of respiratory symptoms [27]. Our study showed a trend of decreased peripheral blood ILC1 and a significantly higher percentage of ILC1 in BALF in TOPD patients compared with controls, as well as a significant increase in the ILC1-associated inflammatory factor, IFN-γ, in both peripheral blood and BALF; therefore, it is reasonable to hypothesize that ILC1 may play a role in the pathogenesis of TOPD, but the exact mechanism remains to be further investigated. In this study, we found that the percentage of ILC2 in peripheral blood was significantly higher in TOPD patients than in the control group and the COPD group. However, there was no significant difference in ILC2 levels in BALF among the three groups. ILC2 secretes granulocyte-macrophage colony-stimulating factor (GM-CSF), which is beneficial for the control of Mycobacterium tuberculosis infection [28]. Further studies are needed to determine whether ILC2 is involved in TOPD pathogenesis after TB infection. Notably, ILC2 responses appear less prominent in COPD, as chronic cigarette smoke exposure can shift ILC2s toward an ILC1-like phenotype [29]; this plasticity may explain the lack of BALF ILC2 differences observed between groups. In addition, ILC1 is closely associated with Mycobacterium tuberculosis. Studies have revealed that ILC1 can enhance its functionality through metabolic reprogramming via glycolysis, accompanied by IFN-γ secretion, thereby directly contributing to the control of M. tuberculosis infection [26]. Its differentiation process is also linked to the early immune responses induced by BCG vaccination, indicating a pivotal role in regulating host antituberculosis immunity.
This study has several limitations. First, its cross-sectional design precludes any conclusions about causality or the temporal sequence of events, limiting our ability to infer definitive mechanisms. Second, we did not perform functional experiments to directly confirm the CXCL13/CXCR5–ILC3 interaction, so the link between this chemokine axis and ILC3 recruitment remains associative. Third, the cohort was relatively small and drawn from a single region with specific inclusion criteria, which may limit the generalizability of our findings to broader COPD populations.
CONCLUSION
In TOPD patients, the percentage of peripheral blood ILC3 decreases, while the percentage of BALF ILC3 increases, compared to controls and COPD patients. Elevated levels of CXCR5, CXCL13, IL-23, IL-17, and IL-22 are observed in both compartments. TB infection may increase CXCL13-CXCR5 axis activity, recruiting peripheral ILC3 to the lungs and potentially limiting airflow. These findings indicate a proinflammatory role for ILC cells in TOPD pathogenesis. Our results suggest that the CXCL13/CXCR5 chemotactic axis may serve as a biomarker of disease severity and a potential therapeutic target for mitigating chronic lung inflammation. Future studies should focus on functional validation of this mechanism and on evaluating targeted interventions aimed at this axis to improve TOPD outcomes.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to the manuscript as follows: F. L., Z.L.: Study conception and design; W. C., X. L., M. C., X. W., Q. L.: Data collection; W. Z., Y. W.: Data analysis or interpretation; Z. L.: Methodology; D, X., J. J., J. W., M. J.: Investigation; W. Z., Y. W.: Drafting of the manuscript. Authorship: All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BALF | = Bronchoalveolar Lavage Fluid |
| COPD | = Chronic Obstructive Pulmonary Disease |
| CXCL | = Chemokine (C-X-C Motif) Ligand |
| CXCR | = Chemokine (C-X-C Motif) Receptor |
| ELISA | = Enzyme-Linked Immunosorbent Assay |
| FCM | = Flow Cytometry |
| IFN-γ | = Interferon Gamma |
| IL-17 | = Interleukin-17 |
| IL-22 | = Interleukin-22 |
| IL-23 | = Interleukin-23 |
| ILCs | = Innate Lymphoid Cells |
| TOPD | = Tuberculosis-Associated Obstructive Pulmonary Disease |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This clinical study was reviewed and approved by the Ethics Committee of the Fourth Clinical Medical College of Xinjiang Medical University, China (Approval Number: 2020XE0131, Date: September 12, 2020).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIAL
The data of current study are available from corresponding author, [Z.L], on a reasonable request.
FUNDING
Funding was obtained from the Central Government Guidance Special Fund for Local Science and Technology Development Project (ZYYD2025QY20) and the Open Project of the State Key Laboratory of High Incidence Disease Causes and Prevention in Central Asia (SKL-HIDCA-2019-ZY16).
ACKNOWLEDGEMENTS
Declared none.


