All published articles of this journal are available on ScienceDirect.
Are Blood Transfusions Useful for Non-Specific Symptoms of Anemia in the Elderly?
Abstract
Background:
Over 10% of adults older than 65 years have World Health Organization defined anemia (Hemoglobin lower than13 g/dl in men and 12 g/dl in women). It is more prevalent with increasing age, exceeding 20% in the very elderly (85 years and older). Typical symptoms of anemia are nonspecific and often attributed to aging or to an exacerbation of another illness in the elderly.
Methods:
We present a case series of patients between ages 65-99 years who were followed at the Senior Health clinic and presented with nonspecific symptoms. All these patients were found to have life-threatening anemia requiring blood transfusions.
Design:
Case series.
Results:
All our elderly patients experienced good outcomes in terms of resolution of their symptoms and improvement in functional status. There was a significant difference in the total number of symptoms pre-transfusion compared with symptoms post-transfusion (p < 0.01). There were no adverse outcomes.
Conclusion:
Our case series suggests that symptoms of anemia in the elderly are often attributed to aging or other disease co-morbidities. Nonspecific symptoms like dyspnea, fatigue and confusion should not be ignored. Management decisions regarding anemia should involve functional assessment of the elderly subject. Immediate arrangements for transfusion must be made if the elderly patient is symptomatic regardless of the hemoglobin level. If monitored appropriately, blood transfusions can prolong survival, improve quality of life and functional status of the older individual.
INTRODUCTION
Prevalence of Anemia in the Elderly
Anemia affects approximately a third of the world’s population and is a major factor in producing functional decline. In 2010, anemia was estimated to be responsible for 68.4 million years lived with disability (YLD) worldwide [1]. The World Health Organization (WHO) defines anemia as hemoglobin (Hb) < 13g/dl in men and < 12g/dl in women. According to this criterion, anemia in the elderly population is often under-recognized and as a result under-treated [2, 3]. The Third National Health and Nutrition Examination Survey (NHANES III) reported the prevalence of WHO defined anemia among community dwelling adults of age 65years and older to be 11% and 10% in men and women respectively. The prevalence of anemia was also observed to increase with advancing age and among the oldest old (85 years and older), it was present in 29.3-30.7% of men and in 16.5-17.7% of women [4-6].
Blood Transfusion Therapy for Anemia in the Elderly
The American Association of Blood Banks (AABB) recommends restrictive transfusion strategy in stable hospitalized patients with transfusion only being considered for individuals with Hb ≤ 7.0 g/dl. Certain exceptions have been made for patients with preexisting cardiovascular disease and for symptomatic postoperative surgical patients where transfusion threshold is higher at Hb ≤ 8.0 g/dl. These recommendations are based only on a few randomized controlled trials that are not representative of many populations including patients with gastrointestinal bleeding, transfusion dependent patients as well as elderly patients recuperating from illnesses requiring hospitalizations [7].
Cumulative clinical experience as well as several studies conducted in the last 10 years suggest that in order to reduce debility, prolonged hospitalizations, medical complications, premature nursing home placement and death in the elderly anemic patients, interventions to correct the anemia may be required at a higher Hb level than that permitted under the restrictive transfusion strategy which has been adopted by most medical centers. There are a number of special considerations that apply in the early recognition of anemia in the vulnerable elderly and need to be emphasized; a) More than 50% of people over the age of 65 suffer from four to five co-morbid conditions often including cardiac or pulmonary diseases that could complicate the diagnosis of anemia with the signs and symptoms being attributed to the other disease conditions; b) Cognitive impairment in the elderly often leads to problems in obtaining a clear history; c) Presentation of diseases is often atypical in the elderly; d) Older people typically have less hemostatic reserve and a rapid deterioration in clinical status can occur if the diagnosis is delayed; e) There might be limitations in conducting an invasive diagnostic work-up in a frail elderly patient; f) The goal of therapy in older cognitively intact individuals is often to maintain their functional independence and improve their quality of life.
METHODS
We present a case series of patients between ages 65-99 years who were followed at the Senior Health clinic between 2007 and 2012. Random number generator was used to select 99 charts from an institutional review board (IRB # 113022) approved data set for seniors with different co-morbid conditions. Charts were reviewed for WHO defined presence of anemia (Hb < 13 in men and < 12 in women). 54/99 patients had WHO defined anemia at some point in a 5 year period.
In-patient and out-patient visits documenting the anemia in these 54 patients were analyzed for the underlying etiology, diagnosis and specific management of anemia. For each case, data collection included problem list, functional capacity, drop in hemoglobin, the level of hemoglobin triggering blood transfusion, pre-transfusion signs/symptoms, hemodynamic instability, functional decline as well as post transfusion benefits or adverse events.
5/54 cases actually demonstrated scenarios of serious or life-threatening anemia presenting with non-specific signs and symptoms where timely blood transfusion averted serious adverse consequences.
CASE #1: Dizziness and Weakness
79 year old African American male with history of Coronary Artery Disease (CAD), Congestive Heart Failure (CHF), Hypertension (HTN), Atrial Fibrillation, status post angioplasty, pacemaker, Implantable Cardioverter Defibrillator (ICD) and Chronic Kidney Disease (CKD) was admitted with complaints of dizziness and weakness after 3 episodes of hematochezia. Patient had undergone a screening colonoscopy a day earlier that had been negative. Admission blood pressure (BP) was 122/71 mmHg (baseline BP 130-140/80-90 mmHg) and heart rate was 88 beats/min. Hb was 12.5 g/dl. After receiving normal saline, patient’s BP returned to baseline but Hb dropped to 10.2 g/dl. The second day patient continued to have hematochezia and his Hb dropped to 8.4 g/dl. The documentation in the chart indicated that patient was hemodynamically stable and asymptomatic at rest. A decision was made to transfuse 2 units of packed Red Blood Cells (RBC) and 2 units of Fresh Frozen Plasma (FFP). Patient’s GI bleeding ceased and Hb increased to 10.5 g/dl. There was no need for any surgical intervention and he was discharged home after observation.
Caveats:
The “hemodynamic stability” noted in this elderly patient’s chart was assessed at rest. This patient was beta-blocked, hence volume depletion and stress-induced responses were likely masked. As per documentation, this patient did not have any presence of hemodynamic instability, nevertheless, timely transfusion in this case very likely averted potential life-threatening cardiovascular consequences and prolonged hospital stay. This case highlights the fact that hemodynamic stability should be assessed appropriately by checking for orthostatic hypotension, tachycardia, weakness, and shortness of breath on exertion because decisions based on assessment of hemodynamic stability in an elderly person at rest could lead to errors in management.
CASE #2: Dyspnea with COPD Exacerbation
76 year old African American male with history of HTN, CKD, Chronic Obstructive Pulmonary Disease (COPD) and Diabetes Mellitus (DM) presented to the clinic with 2 weeks history of progressive increase in shortness of breath and fatigue. He had increased his supplemental oxygen to 3 Liters and was still short of breath at rest. He was unable to do any house work and had difficulty preparing meals. He denied having hematochezia, black stools, chest pain and fever. Vital signs on presentation-BP 168/58 mmHg, pulse: 77 beats/min, respiratory rate: 24 breaths/min. Initial impression was exacerbation of COPD and he was started on Azithromycin and steroids. However, a Complete Blood Count (CBC) obtained for work-up of pneumonia during the visit revealed a Hb of 7.4 g/dl, which had dropped by 2.3 g/dl over the past two months. 2 units of packed RBCs were transfused with a subsequent increase in Hb to 8.7 g/dl. This resulted in a dramatic improvement in his fatigue and functional status although some degree of dyspnea remained. The cause for the anemia was reactivation of an old peptic ulcer which required treatment.
Caveats:
Often shortness of breath can accompany an acute illness or mimic an acute exacerbation of a chronic illness. In elderly patients with multiple serious co-morbidities it can trigger an extensive work-up for exploration of cardiovascular or pulmonary etiologies. In the above case, COPD exacerbation was the initial diagnosis as patient’s presentation was not suggestive of a GI bleed. This scenario also emphasizes that patients with chronic GI bleed may not present with hemodynamic instability when the rate of blood loss is slow and this may mislead the Provider from considering acute anemia among his/her top differentials. Moreover, in the frail elderly, typical symptoms of anemia can easily be falsely attributed to other co-morbidities.
CASE #3: Fatigue, Confusion or Delirium
88 year old Caucasian female with history of HTN, valvular heart disease and transient ischemic attacks developed massive gastrointestinal bleed secondary to Gastric Antral Vascular Ectasia (GAVE) that initially required 6 units of packed red cell transfusions. Subsequently she presented with significant fatigue, dyspnea, weakness, dizziness, with difficulty walking and confusion after recurrent episodes of GI bleeds. Patient usually became symptomatic at Hb values between 7.1-8.7 g/dl requiring packed RBC transfusions every 2-6 months for symptom relief. Several Argon Plasma Ablation procedures were performed to control the bleeding with partial success. Patient’s frequency of GI bleeds increased & her packed RBCs requirement increased to every 5-7 days. For past 6 years patient has remained active and cognitively intact with support from out-patient transfusions with a mean post-transfusion Hb of 10.7 g/dl. She continued to volunteer in the community, limited only by mild fatigue and weakness. It was notable that the patient always developed delirium with a Hb less than 8.0 g/dl. However, because management decisions were always made promptly and efficiently, this patient only required one hospital admission for her symptomatic anemia.
Caveats:
This elderly patient was not a candidate for surgical intervention; packed RBC transfusions for her have been a life sustaining measure. It also highlights the point that timely transfusions with packed RBCs can be done safely in the elderly on an out-patient basis. This is likely cost effective in reducing frequent clinic & emergency room visits as well as hospitalizations for complications related to anemia. Most importantly, this case establishes the fact that in selected transfusion-dependent elderly patients, symptom-driven transfusions can promote functional independence and lead to improved quality of life.
CASE #4: Fatigue, Weakness, Dyspnea on Exertion and Falls
89 year old Caucasian male who had a past medical history notable for HTN, Transient Ischemic Attacks, hypothyroidism, dyslipidemia, Restless leg syndrome, hereditary spastic paresis, status post laser prostatectomy, urinary incontinence, status post dilatation of urethral stricture, recurrent bladder/renal stones and intermittent hematuria presented to the clinic with 2 week history of progressively worsening fatigue, weakness, falls and mild dyspnea on exertion. Patient’s CBC from 3 days prior was notable for Hb of 7.3 g/dl; a drop from 11.4 g/dl three months earlier. He was seen in the clinic and was sent to the hospital for urgent blood transfusion. He reported recurrent hematuria but his urinalysis showed only 3-5 RBCs. Patient denied recent history of hematochezia, melena and hematemesis. On admission, his Hb was further reduced to 6.7 g/dl with greater weakness and shortness of breath. On documentation, he remained hemodynamically stable at rest. He required three units of packed red cells and Hb the next day at discharge was 8.9 g/dl. Patient’s symptoms of dyspnea and weakness resolved after receiving the blood transfusion with only residual mild fatigue.
Caveats:
This is a frail older patient with multiple chronic co-morbidities. His constellation of symptoms was not very prominent because of his limited physical function and even while in hospital he remained “hemodynamically stable”. What triggered an evaluation was his falls, which could be attributed to the spastic paralysis but had become more frequent. This patient did not want a GI work-up at that time, so the cause of anemia could not be determined. The transfusions were probably life-saving and significantly improved his quality of life and function.
CASE #5: Weakness and Shortness of Breath
79 y/o Caucasian male with past medical history notable for HTN, Alzheimer’s dementia, dyslipidemia, depression, Gastroesophageal Reflux Disease, Obstructive Sleep Apnea (OSA) and recurring GI bleeds presented to the Emergency Room with weakness and black stools. His BP and pulse were 80/43 mmHg and 82 beats/min respectively and Hb level was 8.8 g/dl on presentation. Patient’s symptoms resolved and BP stabilized after 3 units of packed cell transfusion. Hb recovered to 12.7 g/dl post-transfusion. Except for minimal esophagitis and villous adenoma, Esophagogastroduodenoscopy (EGD) and colonoscopy were unrevealing. Three months prior to this episode the patient had presented with significant shortness of breath, vomiting and diarrhea and a history of black stools. He was afebrile on presentation with a BP of 104/52 mmHg. Hb was 12 g/dl and White Blood Cell (WBC) count was 19,000/mm3. CT chest, abdomen and pelvis were unremarkable and WBC scan did not reveal any abscess. Patient’s lowest recorded Hb during that admission was 8.9 g/dl and he received a unit of blood. Weakness and shortness of breath resolved as Hb improved. EGD showed a Mallory Weiss tear, severe esophagitis and a duodenal ulcer.
Caveats:
It is noteworthy that this patient presented with acute anemia secondary to GI bleed that was symptomatic at a hemoglobin level greater than the recommended transfusion threshold. Also, transfusion averted significant morbidity and led to a short hospital stay. This patient was cognitively impaired and did not complain of gastric/ esophageal pain, however, a complete work-up revealed a reversible, life-threatening cause of anemia and timely transfusion averted a worse outcome.
Statistical Analysis
The difference in hemoglobinpre and post-transfusion was significant with p < 0.03 (Mann-Whitney U test), as shown in Fig. (1). The total number of non-specific symptoms experienced by the patients pre-transfusion was 19 and post-transfusion was 5, which was also significantly different with p < 0.01 (Mann-Whitney U test), as shown in Fig. (2).
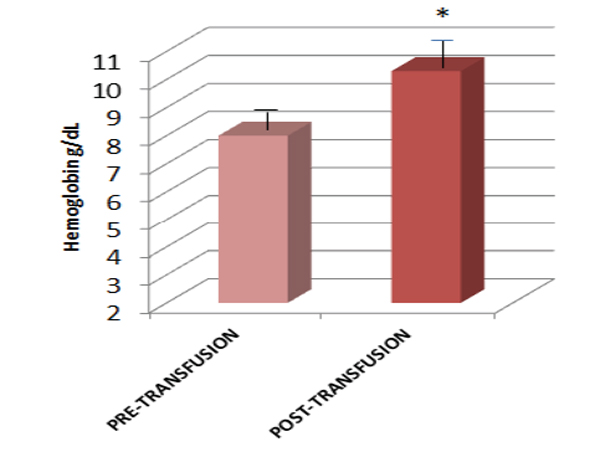
Hemoglobin value in anemic subjects before and after blood transfusion.

Symptom score in anemic subjects before and after blood transfusion.
DISCUSSION
The cases described above illustrate the point that AABB transfusion guidelines cannot be adopted as a standard for all anemic patients especially the geriatric population in whom serious anemia often presents with non-specific or vague symptoms requiring transfusion at a higher hemoglobin level than recommended. Better recognition of the signs and symptoms of anemia is needed in the elderly in whom symptoms are often attributed to aging or other disease co-morbidities. Symptomatology and severity of anemia can be masked in the elderly because of a reduced level of activity. Management decisions based on signs and symptoms of anemia should involve functional assessment of the elderly subject.
In 2000, there were 35 million people over the age of 65 in the United States. This age group is expected to increase to 80 million by year 2050. The greatest increase of approximately 5 foldis expected in the age cohort of 85 years and older. Thus the impact of chronic diseases and associated disability in the elderly will have great implications for healthcare in the coming decades. Anemia is now being recognized as one of the common conditions that must be addressed more vigorously, both in the out-patient and in-patient environment, to reduce the burden of morbidity and mortality and improve quality and quantity of life of the aging strata of our society. In the past 2 decades a number of studies have been conducted to assess the impact of anemia on the elderly (Table 1), and the major findings of some of these studies are discussed below.
Studies on impact of anemia in elderly.
| Conditions Associated with Anemia | Study | Type & Duration of Study | Sample Size & Age | Methodology | Results |
|---|---|---|---|---|---|
| Delirium | Joosten et al.Belgium2006 a | Longitudinal prospective study over 5 months |
190 elderly patients in acute geriatric ward. Mean age of the group was 82.5 yrs |
Study patients investigated with Mini Mental Status Exam (MMSE), Confusion Assessment Method (CAD), demographic, clinical and lab data |
18% of the patients identified by delirium. 50% of patients had anemia. Anemia, male sex, age > 82 yrs, dehydration and dementia were independent risk factors for delirium. |
| Axell et al.Sweden2002 b | Longitudinal prospective study: Data collected over 6.5 months |
19 ventilated patients in Intensive Care Unit (ICU) Median age 70 yrs |
Study patients had an ICU stay length of > 36 hrs and were closely observed during the stay. Patients were interviewed in depth twice after discharge. |
6 patients had severe delirium & 8 moderate delirium. Severity of delirium was associated with the degree of anemia. Patients with severe delirium had significantly lower Hb (7.5-9.5g/dl). Patients with severe delirium required higher doses of fentanyl and midazolam and a longer duration of ventilation & ICU stay. |
|
| Dementia | Hong et al. S. Korea& USA2013 c | Prospective Cohort Study: data collected over 11 years of follow up |
2552 community dwelling, ethnically diverse. Mean age: 76.1 yrs. Memphis TN & Pittsburgh Pennsylvania. |
Cognitive function assessed at 1,3, 5, 8,10, 11 years with a global test |
2552 subjects; 51.8% female, 38.9% were black. 393 (15.4%) had anemia at baseline and 455 (17.4%) developed dementia. Older adults with anemia at baseline had a higher risk of developing dementia (22.7%) as compared to those who did not have anemia (17%) After adjusting for age, race, sex, education, literacy, APOE ɛ4 status, mini-mental status exam, co-morbidities (stroke, HTN, DM and Myocardial Infarction), renal function, indices of anemia indices & C-Reactive Protein, the results remained statistically significant. |
| Shah et al. IL USA2011 d | Prospective study: participants followed for 3.3 yrs | 881 community dwelling: Mean age; 80.1 yrs Chicago, IL. | Cognitive decline assessed over a period of 3 years in participants of the Rush Memory and Aging project who did not have dementia. |
Follow up over a period of approximately three years was notable for development of dementia in 113 patients. Those who developed dementia were of older age and had poorer performance on MMSE. Their BMI and cognitive function were also relatively lower. In comparison to individuals without dementia, those with dementia were more likely to be depressed and had a smaller social circle. Subjects with Hb of 13.7 g/dl had the smallest rate of cognitive decline. Anemic participants had a faster decline in cognitive function in comparison to those without anemia. |
|
| Atti et al.Sweden & Italy2005 e | Prospective study (Kungsholman project); Subjects' data analyzed at baseline and at 3 years. |
1435 subjects without dementia. Age range: 75-95 years |
Comparison of data at baseline to 3 year follow up. |
At the end of 3 years, anemic patients with normal cognition had a two times higher risk of developing dementia than cognitively intact non-anemic patient after adjustment for gender, age and education. The association between anemia and development of dementia remained unchanged even after adjusting for chronic disease, inflammation and nutritional status. 189 subjects were diagnosed with dementia on follow up at 3 years; 77.2% were diagnosed with AD and 12.7 % with Vascular dementia. After adjusting for age, gender and level of education, subjects with anemia were two times more likely to develop AD. |
|
| Decline in Physical Performance | Thein et al.USA2009 | Multi-center cross-sectional survey performed over one year. | 328 patients: mean age 76.8 year | Three sites participated. Instruments used: SF-36 & Functional Assessment of Chronic Illness Therapy-Anemia (FACIT-An), IADL, GDS & Handgrip strength. Questionnaires and measurements performed within 1 month of serum Hb measurement. |
68% were women; age ranged from 65-103 yrs Older patients (75 yrs and above) had a lower mean Hb level (12.8 g/dl) in comparison to the younger (age range 65-74 yrs) patients whose mean Hb level was 13.3 g/dl. 90 (27%) patients had WHO defined anemia: 54 females and 36 (31%) males. There was almost a 10 point difference in the unadjusted mean SF-36 scores of patients with Hb of 15 g/dl (47.1) and those with Hb less than 12 g/dl (37.3). This decreasing trend was evident across all Hb groups as well as the five physical function subscales. |
| Penninx et al. Italy2004 f | Cross-sectional study over18 months | 1008 participants: mean age 75.4 years | Debility in 6 activities of daily living & 8 instrumental activities of daily living. SF36, knee extensor and handgrip strength assessments. | Men had an average Hb level of 14.4 g/dl and women's mean Hb level was 13.2 g/dl. 11.3% of the participants were anemic. Subjects with anemia had lower scores on SF 36, decreased handgripand knee extensor strength when compared to subjects without anemia. | |
| Penninx et al.USA2003 | Prospective cohort study over 4 yrs. | 1146 subjects: mean age: 77 yrs | Evaluation of balance, a timed 2.4-m walk, and a timed test of five chair rises for assessment of physical function; these were incorporated into scale: 0 (poor) to 12 (excellent) |
There was a significant association between anemia and mean decline in physical function over a period of 4 years; 2.3 in anemic subjects and 1.4 non-anemic subjects (P = 0.003) This association between anemia and increased functional decline was also seen in participants without chronic illnesses associated with anemia (malignancy, infectious disease and renal insufficiency) and after adjusting for lipid, iron and albumin levels. Subjects with borderline anemia, a Hb level within 1 g/dL over the WHO defined anemia, also showed a higher mean physical decline (1.8) vs those with greater Hb levels (P = 0.02). |
|
| Hospital-ization & Mortality | Culleton et al.Canada2006g | Prospective cohort study over 3 yrs | 17030 community dwelling subjects: ≥ 66 yrs | Cox potential analyses performed to assess associations between anemia (defined as HB less than 11 mg/dl) and hemoglobin and all-cause mortality. |
1983 mortalities and 7278 first hospitalizations were recorded. Analysis showed a higher risk of mortality, first all-cause hospitalization, and first hospitalization for cardiovascular causes (HR, 2.49; 95%) in anemic patients with normal kidney function. The association between Hb and all-cause mortality was evident as an inverse J-Shaped curve. Women with Hb level 13-15 mg/dl and men with Hb level between14-17 mg/dl had the smallest risk for mortality. |
| Riva et al.Italy2009h | Prospective population based study over 4 years | 7536 elderly with mean age between 73.6-74.9 yrs | Data from 7536 elderly utilized to determine mortality. Complete health information available for 4501 subjects. | There was an increased risk of hospitalizationand mortality in the elderly subjects with mild anemia versus subjects who did not have anemia. | |
| Denny et al.NC USA2006i | Prospective cohort study | 1744 community dwelling elderly: mean age: 78 yrs | Hb assessed at baseline. Physical performance, using Katz, IADL and cognition using Sort Portable Mental Status Questionnaire were assessed at baseline and 4 year follow up interview. |
33% of women and 31% of men had anemia. Anemic subjects were of older age more often had African American ethnicity, were less educated, had suboptimal kidney function and a hospitalization in the previous year. Caucasians in all age cohorts assessed had a higher Hb level in comparison to their African American age cohorts. A statistically significant rise in mortality was observed in all subjects with anemia in comparison to those without anemia. This was unchanged even after adjusting for sex. At 8 years, women with Hb level between 13 and 14 g/dl men with hemoglobin level between 14 and 15 g/dl had the greatest survival percentage. The lowest rate of survival was notable in Hb levels at both extremes. |
|
| Penninx et al. USA2006j | Longitudinal prospective study over 4 years | 3607 participants of EPESE study with mean age of 78 years | Anemia as per WHO criteria. Data regarding death and hospital admissions over 4 years accessed from death records and the Medicare database. |
12.5% of the subjects had anemia. Participants with anemia had a greater likelihood of death and hospitalization than those without anemia in the follow-up period. After adjusting for demographics and co-morbid illnesses, anemia was found to have statistically significant association with subsequent death and hospitalization. Anemia alsoshowed a statistically significant association with mortality and hospitalization in participants without co-morbidities at baseline. |
|
| Mortality | Elzen et al.Netherlands2009k | Longitudinal prospective study (Leiden 85-plus Study): patients followed over 5 years | 562 people Age: 85 years | Anemia per WHO definition. 3 aspects of functional state assessed at baseline and once every year for 5 years: debility in basic and instrumental activities of daily living, cognitive function and presence of low mood. Data on death accessed from the municipal registry. |
26.7% of the elderly participants had anemia at baseline. At baseline, anemic subjectshad greater debility in physical function, lower cognitive function and more symptoms of depression than the non-anemic participants. These differences were not significantafter adjustment for comorbid illnesses. On subsequent follow up, anemia was associated with a decrease in Instrumental Activities of Daily Living, which remained significant after adjusting for comorbid conditions. Subjects in whom new onset of anemia was found on follow up (n = 99) also had an increase in disability. Both categories of anemia patients, new-onset and previous diagnosis, had higher mortality rates after adjusting for various demographical variables including education level, gender, socioeconomic status, and other variables (living in long-term care facility, CRP level, creatinine clearance and disease status) |
Letters referring to numbered references in text as follows:
a = 16
b = 17
c = 19
d = 21
e = 20
f = 23
g = 8
h = 2
i = 9
j = 10
k = 11
Anemia and Mortality
Anemia amongst older adults (over the age of 65) also increases the risk of hospitalization and mortality. A recent large Canadian study of over 17000 older adults (66 years and older) showed a significant association between anemia and risk of hospitalization secondary to all causes, including cardiovascular related hospitalization and all-cause mortality [8]. Likewise a European study of 4500 adults between ages 65-84 years also revealed increased likelihood of hospital admissions and all-cause mortality of anemic patients versus non-anemic patients after controlling for many possible variables [2]. Other prospective studies from Europe and USA have also shown a statistically significant association between anemic elderly and increased risk of hospitalization and mortality [2, 9-11]. Among hospitalized anemic patients, studies have shown an increased likelihood of delirium, mobility disability and falls that often results in prolonged in-patient course.
Anemia and Delirium
Delirium, a clinical syndrome that is defined as inattention and acute cognitive dysfunction affects an estimated 14-56% of all hospitalized elderly patients [12-18]. At least 50% of geriatric patients hospitalized annually in the US develop complications secondary to delirium with a mortality rate between 25-33% [12, 13].
Among older surgical patients, post-operative delirium is seen in 15-53% and its incidence is as high as 70-87% in elderly Intensive Care Unit patients [14]. Delirium is associated with worse outcomes, extended hospitalizations, higher costs, cognitive decline and increased mortality rates [12-15]. Some of the important risk factors for delirium in the elderly are dementia, male sex, high burden of co-morbidities, certain medications, sensory impairment, sleep deprivation, metabolic derangements and pain [14]. Recent available studies have shown that anemia significantly and independently increases the likelihood of delirium in the ward, medical & surgical ICUs as well as post-operative settings. In our particular case study (subject # 3), the patient only developed delirium when Hb dropped below 8 g/dl, but she was otherwise cognitively intact and had age-appropriate functional capacity. It is likely that patients with some element of dementia or hypoxemia could develop confusion at higher Hb levels.
In a small 5 month prospective study of 190 elderly patients (mean age, 82 years) performed in a Belgian hospital geriatric ward, 18% were identified with delirium. It was significantly more frequent among male patients in comparison to female patients. Results showed maximum number of delirious patients (among both men and women) in the quartile with lowest Hb i.e. < 10.8 g/dl. Statistical analysis with adjustment for major confounding factors showed anemic men but not anemic women to have a high likelihood of becoming delirious and this likelihood increased with decreasing Hb concentration [16]. Similarly another study conducted in a Swedish hospital ICU showed that patients with moderate and severe delirium had lower Hb concentrations (6-9.5 g/dl) in comparison to those with no symptoms of delirium [17].
Anemia was also found to independently increase the risk of delirium after cardiac surgery in a recent Polish study [18]. The authors postulated that factors such as atrial fibrillation and hypoxia were the likely cause of cerebral hypoxemia, inhibiting the formation of neurotransmitters and resulting in decreased cholinergic and glutaminergic activities in the cerebral cortex resulting in delirium [18].
Anemia and Dementia
In the last decade there has been mounting evidence linking anemia to the risk of developing dementia. The most recent prospective cohort study investigating this link was published in August 2013 and involved 2552 community dwelling older adults. Participants were followed over a period of 11 years. Cognitive function was evaluated at years 1, 3, 5, 8, 10 and 11 with Modified Mini Mental Status (3MS) examination. Dementia diagnosis was established by review of hospital records, prescription of dementia medication or a race stratified decline in 3MS score of more than 1.5 SD from baseline mean. 15.4% (393/2552) of the subjects had anemia at baseline. Over 11 years, 17.8% (455/2552) were diagnosed with dementia. Older adults with anemia at baseline were more likely to develop dementia (n = 89, 22.7%) compared to those who were not anemic (n = 366, 17%) (p = 0.007). This association between anemia and incident dementia retained its statistical significance after accommodating for various potential confounders (age, sex, race, education, literacy, APOE ɛ 4 status, 3MS score, and co-morbidities: stroke, HTN, DM, MI, anemia parameters: MCV, RDW, erythropoietin, renal function and CRP) [19]. Two other prospective studies have also shown a statistically significant association between anemia and risk of developing dementia [20, 21].
The mechanisms explaining this association have not been well delineated to date. However, several hypotheses have been suggested including impairment of cerebral perfusion and a sudden decrease in oxygenation of the brain leading to a poor cognitive performance. In chronic anemia, poor cerebral oxygenation places the individual at increased risk of developing dementia [20]. The role of erythropoietin in the pathogenesis of dementia is also an area of developing interest. In animal models of stroke and hypoxia, erythropoietin has been shown to have a protective effect. Hence, it has been hypothesized that lower erythropoietin levels as seen in chronic kidney disease may enhance the likelihood of neurodegenerative process. Iron and vitamin B12 deficiencies have also been thought to contribute to poor oxygenation of the brain and cognitive decline [19].
Anemia and Decline in Physical Function
Mobility impacts functional independence. When mobility disability (difficulty performing such tasks in one’s own environment) occurs in the elderly, it inadvertently leads to loss of autonomy, institutionalization and mortality. Anemia is also a significant predictor for functional decline. Recent data from 633 communitydwelling women, from the Woman’s Health and Aging Study (WHAS) I & II showed that even with mild anemia there was an increase in mobility disability risk [22]. In the WHAS, similar findings were also observed between Hb levels and objective mobility performance as assessed by the Short Physical Performance Battery (SPPB) index. Results showed that the proportion of older women with best SPPB scores had the highest Hb (13-13.9 g/dL) and those with the worst scores had Hb concentration in the range 10-12 g/dl.
Further, a prospective study of 1146 older candidates (mean age, 77 ± 5 years: 70% female) participating in the Established Populations for Epidemiologic Study of the Elderly (EPESE) reported that older men and women with anemia experienced clinically significant mobility decline as compared to their non-anemic counterparts, even after adjustment for major confounders [22].
Studies have also shown that anemia interacts with co-morbidities that are common in older adults to synergistically increase the risk of outcomes such as frailty and mortality. The independent contribution and impact of anemia on frailty was investigated in a cross-sectional study that examined data from the Women’s Health and Aging Study (WHAS) I &II. In this study, definition of frailty was as pre-established criteria with presence of 3 of 5 manifestat-ions: shrinkage, slow gait speed, weakness, exhaustion and decreased physical activity. The relationship between frailty and anemia was observed as a reverse J curve comparable to the documented association of anemia with mobility-disability and mortality. The lowest incidence of frailty was observed at mid-normal Hb levels around 13.5 g/dl. Frailty was 2.8 times more likely at Hb level of 11 g/dl and 1.5 times more likely at a Hb level of 12 g/dl versus 13.5 g/dl.
The link between anemia and muscle strength was analyzed in a cross-sectional study that studied data from 1008 community dwelling older men and women, age 75.4 ± 7.3 years participating in the Aging in the Chianti Study. In this study, older adults with anemia had significant decrease in grip and quadriceps strength even after adjustment for major confounders. A positive relationship has been shown to exist between hemoglobin levels and muscle strength, especially knee extensors and grip strength [22].
In all of the cases that we presented, patients were discharged home fairly rapidly upon improvement of their symptoms although an appropriate assessment of physical function pre-discharge was not done. It is understandable that fatigue and weakness takes time to abate and supplementation with oral iron therapy helps achieve that goal. It is important that the functional status of an older person be assessed not only pre- and post-transfusion, but also periodically during their rehabilitation, since anemia can adversely impair the process of recovery. A hypothesis supporting the causal association between anemia and unfavorable functional outcomes suggests that anemia might limit the maximal and sub-maximal aerobic exercise capacity resulting in exercise intolerance or behavioral modifications (such as decrease in physical activity) that further promotes deconditioning. In turn, anemia-related chronic physical deconditioning could contribute to decrease in muscle mass, strength and quality (sarcopenia and dynapenia), osteoporosis, autonomic dysregulation, executive dysfunction and accelerated renal functional decline. At the cellular level, anemia decreases the oxygen carrying capacity of the blood with chronic tissue hypoxia, and limits the homeostatic responses to acute and chronic stressors, which is the hallmark of frailty [23, 24].
Common Types of Anemia in the Elderly
According to the data from NHANES III, anemia among older adults in the United States is attributable to nutritional deficiencies (Iron, Vitamin B12 and Folate) in 34%, chronic diseases in 20%, and remains unexplained despite exhaustive work up in 34% of the cases [25]. Among the nutritional group, iron deficiency anemia (IDA) by itself or concomitantly with folate or B12 deficiency constitutes more than one half of this group [26].
Etiology of IDA among elderly in the developed world is less often secondary to inadequate dietary intake and more frequently from chronic gastrointestinal blood loss [25]. In 40-60% of the patients, the etiology is the upper GI tract (related to Peptic Ulcer Disease (PUD), esophagitis, gastritis, NSAIDs use, and angiodysplasia) and in 15-30% it is the colon (lesions being colorectal cancer, premalignant polyps &angiodysplasia). Dietary inadequacies, Helicobacter pylori infection, malabsorption secondary to atrophic gastritis and rarely celiac disease account for the remaining percentage of IDA in elderly population [27].
Approach Towards the Diagnosis of Iron Deficiency Anemia in the Elderly
History:
When evaluating a person for iron deficiency anemia, it is important to inquire about any known history of PUD, bleeding disorders, celiac disease, Abdominal Aortic Aneurysm and gastric bypass surgery. Patients’ dietary habits should also be assessed. Strictly vegan diet can result in iron deficiency as iron from meat, poultry and fish, all sources of heme iron, is absorbed two to three times more efficiently than iron from plant sources (fortified cereals, some beans and spinach). It is also important to know about patients’ alcohol, tea/coffee consumption. Excessive alcohol intake can result in iron deficiency anemia by inhibiting binding of iron into heme molecules of Red Blood Cells and by potentially causing GI blood loss [28]. Coffee and tea contain tannins and polyphenols that decrease iron absorption in the gut, hence, increase intake of these beverages contributes to IDA [27].
Physical examination:
IDA often presents with nonspecific signs and symptoms. A patient with IDA is likely to report varying degree of dizziness, light-headedness, easy fatigability, tiredness or weakness. Depending on severity, anemia can manifest as postural hypotension, tachycardia, pallor, koilonykia, decreased muscle strength, headaches and/or confusion. In addition, the signs and symptoms of anemia in the elderly are often masked by other co-morbid conditions common in this population, which make the diagnosis more challenging. Some common conditions that can be mistaken for anemia or are frequently encountered co-morbidities in the elderly are listed in Fig. (3). Adverse effects of medications should also be considered in all older patients because of issues associated with polypharmacy and the erosive effects on the gut of some over-the-counter drugs such as NSAIDs. It is also important that vital signs and the cardiopulmonary responses of the patients are determined during activity as well as rest. An older person, who might appear hemodynamically stable in bed, could have chest pain, shortness of breath, tachycardia and severe weakness upon arising from bed and walking around the room (Fig. 3). This would call for a re-evaluation of the decision to transfuse the patient, who otherwise might have been discharged in a borderline unstable condition. Hence, the importance of a functional evaluation in an older adult cannot be over-emphasized.

Physical and functional assessment of iron deficiency anemia in an older adult.
CBC with hemoglobin < 12 g/dL in women and < 13 g/dL in men.
Laboratory tests:
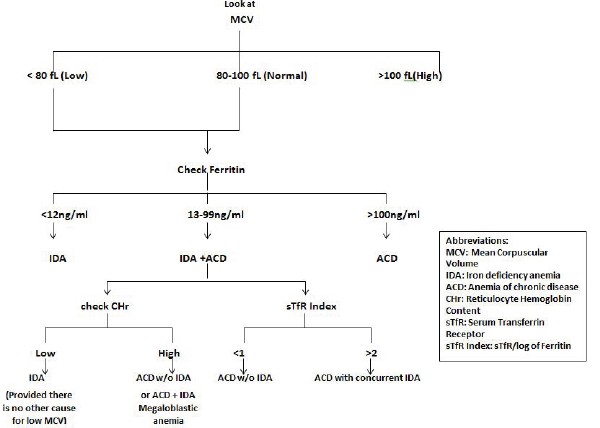
There are several laboratory investigations for assessing iron status in patients but diagnosing Iron deficiency in the elderly is often a challenge as most of the available tests have limitations. There are many algorithms for the laboratory diagnosis of anemia which can often be very complex. Since our focus is on iron deficiency, we have provided a simple outline for the laboratory evaluation of elderly patients (Fig. 4).
Mature Erythrocyte Indices
RBC size:
This is an important nonspecific marker for nutritional anemias [26]. In the elderly, however, because of multifactorial etiology, it does not always aid in diagnosis. A recent study showed only 27.5% of patients with IDA to have microcytosis and just 7.4% of patients with folic acid and/or cyanocobalamin deficiency to have macrocytosis [29]. In pure iron deficiency anemia RBC is microcytic (MCV < 80) whereas when anemia is only due to inadequate folate and/or cobalamin levels, it is macrocytic (MCV > 120) [27, 30].
Red blood cell distribution width (RDW):
A measure of heterogeneity in red blood cell volume distribution is also considered a sensitive indicator of IDA (sensitivity 81% and specificity 53.4%) [31].
Markers of Iron Stores, Uptake and Metabolism
Serum Ferritin: is considered by many to be the best test available for detecting iron deficiency [31-33]. It reflects iron stores and decreases in iron deficiency anemia. However, ferritin is also an acute phase reactant and its elevation is influenced by inflammation (chronic infections, autoimmune diseases, malignancy, etc.). In certain inflammatory conditions such as RA, CHF and CKD, ‘functional iron deficiency’ occurs whereby there is iron-restricted erythropoiesis despite adequate iron stores causing Ferritin level to be either normal or elevated. This is a condition frequently termed Anemia of Chronic Disease (ACD) [34]. Functional iron deficiency is thought to be modulated by Hepcidin, a small polypeptide, produced by the liver that acts as a direct mediator of iron homeostasis. It also acts as an acute phase reactant and is elevated in the presence of pro-inflammatory cytokines. Increased levels of hepcidinprevent gastrointestinal absorption of iron, release of iron from the hepatic reserves and from macrophage/ monocytes to erythroidproginator cells [34, 35].
However, Ferritin levels ≤ 12 µg/dl are diagnostic of iron deficiency and levels ≥ 100 µg/dl reliably rule out iron deficiency. Levels between 13-99 µg/dl remain an area of undetermined significance [36].
Transferrin Saturation (TSAT): is the percent of iron bound transferrin. It is the ratio of serum iron and total iron binding capacity, multiplied by 100. The normal level is between 20%-50%. Iron deficiency, if not complicated by concomitant inflammatory disease, will yield a low TSAT (< 20%). TSAT also behaves as an acute-phase reactant as transferrin may be high in inflammatory states. This would result in a lower TSAT if serum Iron stays constant. A TSAT of 20% has a sensitivity between 81-88%, and iron deficiencyanemia with a TSAT higher than 20% is uncommon [37, 38].
Serum Transferrin Receptor (sTfR): is a transferrin receptor (TfR) that is localized on the surface of erythroid precursors [39]. It is considered to be a sensitive alternative for assessment of iron stores with normal levels being between 2.6-9.9 mg/dl. sTfR has an inverse relationship with tissue iron stores and increased levels are indicative of iron deficiency [33]. Decreased levels of sTfR are present in hematologic conditions such as erythroid aplasia, erythroidhypoplasia as well as in conditions of severe iron overload. sTfR levels are elevated in patients receiving erythropoietin therapy or in those with hemolytic anemias, erythroidhyperplasiasor severe megaloblastic anemia [39].
sTfR Index: The ratio of sTfR to log of serum ferritin (sTfR/log Ferritin) is a highly predictive test for the estimation of iron stores in the body. However, it is not a commonly performed test in clinical laboratories [32]. The sTfR Index is useful in the presence of ACD as suggested by elevated C-reactive protein [39].
Zinc Protoporphyrin/Heme Ratio: identifies depletion of iron stores prior to development of anemia. It is a predictor of iron status in the bone marrow during erythrocyte production. When iron is inadequate, zinc movement across the intestinal membrane is increased to substitute for the missing iron in protoporphyrin ring production. This test has limited specificity as zinc protoporphyrin rises in conditions such as inflammation, ACD and hemoglobinopathies [31].
Reticulocyte Indices
Reticulocyte Hemoglobin Content (CHr):
The CHr provides an indirect assessment of the availability of functional iron for erythropoiesis over the past three to four days [31, 33]. Thus CHr has been useful in detecting functional iron deficiency that is present in ACD and hence need for iron supplementation [35]. Low CHr is also seen in the absence of iron deficiency in certain conditions such as hemoglobinopathies (thalassemia) that produce microcytic anemia. Similarly, CHr may be increased in patients with megaloblastic anemia and iron deficiency [35, 40, 41].
Bone Marrow Biopsy
If the diagnosis of anemia remains unclear after extensive lab testing, a bone marrow biopsy may be performed. The gold standard test for the diagnosis of iron deficiency anemia is the absence of stainable iron in the bone marrow.
Gastrointestinal (GI) Evaluation
This is extremely important in investigating iron deficiency anemia in older adults, as almost 60% of IDA patients over the age of fifty have a gastrointestinal source.
Fecal Occult Blood Testing (FOBT): is used commonly to detect blood loss in the stool that is not visible on gross inspection. It is usually less than 50 mg of Hb per gram of stool. Normal adults usually lose less than 2 to 3 mg/g of stool [42]. Blood loss of at least 10 milliliters per day is needed to give a positive result on this test. In men over the age of 50 and postmenopausal women, the use of this test is discouraged since irrespective of the FOBT result upper and lower GI endoscopy is strongly recommended if bleeding is suspected [33].
Upper and Lower GI evaluation: is recommended in all postmenopausal women and men over the age of 50 who have a confirmed diagnosis of IDA in the absence of overt non-GI blood loss [9]. There is little consensus on the degree of anemia warranting investigation. In the elderly individuals with iron deficiency, GI evaluation is recommended irrespective of the Hb level since a similar prevalence of GI lesions was observed in iron depleted patients without anemia as in those with anemia [31, 43].
GI evaluation comprises endoscopic testing including EGD, Colonoscopy, enteroscopy and wireless capsule endoscopy (WCE) and radiographic tests: barium enema, upper GI series with or without small bowel follow through, enteroclysis, abdominal Computer Tomograpy (CT) and CT colonography. The choice or appropriateness of test depends on multiple factors which include: type & location of the lesion, patient’s condition (frailty, severe co-morbidities) and availability of test, presence of contraindications to a particular test and potential fitness of the patient to tolerate treatment in the event a colorectal cause is identified. However, Endoscopic modalities are diagnostically superior to radiographic tests as they allow direct visualization of lesions, and often treatment (resection of polyps/adenomas) and performance of biopsies. Nevertheless all tests have their limitations and may not be appropriate in all situations [31].
Those over the age of 50 with marked anemia or with family history of colon cancer should still undergo lower GI investigation even if they have been found to have celiac disease [32]. Approximately, 5-10% of patients with anemia have no lesions found on endoscopic evaluation with EGD and colonoscopy. According to the American Gastroentero-logical Association (AGA), patients who have persistent or recurrent IDA after negative EGD or colonoscopy should undergo small bowel evaluation and WCE should be the desired method for examining the small bowel [31].
Non-Transfusion Therapy of Iron Deficiency Anemia
If the patient is stable and asymptomatic then iron should be replaced orally with dietary supplementation. As a first step, assessment of the patient’s diet should be undertaken with recommendations to include iron rich foods (i.e. fortified cereals and breads, red meat, beans, green leafy vegetables). It may be beneficial to get a registered dietitian or a licensed nutritionist to assist in the proper education of required dietary changes. Often diet alone is inadequate to correct the anemia and oral iron therapy is required.
Oral Iron Therapy: is the first line therapy for IDA. It is cheap, effective, safe and convenient [31, 33]. Ferrous sulfate, ferrous fumerate and ferrous gluconate are the available oral forms and are usually prescribed as twice daily dosing in the elderly. It is, however, important to know that a single tablet of ferrous sulfate contains twice the amount of elemental iron as a tablet of the other two formulations. Also all three formulations are available in enteric coated and non-enteric coated forms. The enteric coated-delayed release preparations may be better tolerated but are less effective as they have less elemental iron [44]. Patients who do not show an adequate response to enteric coated-delayed release formulations may show a good response to non-enteric coated ferrous preparations.
Iron is better absorbed in acidic environment (in the ferrous versus ferric form) and its efficacy can be further optimized by taking it with orange juice or ascorbic acid tabs (250-500 mg twice daily) [31, 32, 44]. Foods (tea, coffee, phosphate containing carbonated beverages like soft drinks) and medications (antacids, proton pump inhibitors and H2 blockers) that inhibit gastric acid secretion must be avoided at the time of iron therapy administration. Oral iron therapy should generally be taken 1-2 hours after taking an antacid. The acid suppressive effect of H2 blockers and proton pump inhibitors is even more extended. Hence, iron tablets should be taken at bedtime when the alkalizing effect of food isminimum and gastric acid production is active.
Often upper (epigastric discomfort and nausea) and/or lower (constipation and diarrhea) GI side effects develop on oral iron therapy. These can be managed by dose reduction (prolonging dosing intervals) or changing the iron formulation [44]. An increase of 2 g/dl in the hemoglobin level is considered to be an adequate response to oral supplementation [34]. Oral Iron Therapy should be continued for 2-3 months after anemia resolution in order to restore the body’s iron stores [45]. Once IDA resolves, it is recommended to perform a 3 monthly evaluation of Hb and red cell indices for one year, then after a further year and again if symptoms of anemia develop after that [32].
Intravenous Iron Therapy:
Three main indications for parenteral iron therapy are:
- Failure of oral iron therapy: often patients are not able to tolerate the side effects (constipation, nausea, vomiting and abdominal discomfort) or fail to be complaint with oral therapy.
- Iron malabsorption occurs in malabsorption syndromes such as Celiac disease (now increasingly recognized in elderly individuals), conditions of decreased gastric acid secretion as in atrophic gastritis or with H2 blockers and Proton Pump Inhibitors (PPI) or in the event of partial gastrectomy or gastric stapling for obesity.
- High iron requirements: in situations of excessive GI bleeding or in chronic hemodialysis, the iron absorbing capacity of the gut is overwhelmed.
Intravenous iron is available in 4 forms: iron dextran; this was the only available formulation in the United States until recently. It can be administered in large doses on a single occasion but the dextran moiety has the potential to cause serious anaphylactic reaction. Iron gluconate and sucrose have recently been approved in the US: these formulations do not carry the serious threat of potentially fatal allergic reaction but can only be administered in limited doses [46]. Ferric carboxylmaltose is a new intravenous iron formulation that can be given safely in high single weekly doses at much higher infusion rates than other parenteral iron formulations [31].
While administering intravenous iron therapy, patient must be watched closely for any signs/symptoms and also evaluated for laboratory evidence of iron overload. Serum ferritin > 800-1000 µg/dl, transferrin saturation more than 50% or abnormal liver function tests are indicators of iron toxicity. Parenteral iron administration must be stopped if these levels are exceeded otherwise there is a high risk of iron deposition in the organ such as the heart, liver and pancreas [46].
CONCLUSION
Anemia is a significant problem in the elderly and it should not be considered an inevitable consequence of aging. Over the last 10 years multiple studies have outlined the adverse acute and long-term consequences of anemia such as delirium, weakness, immobility, falls, prolonged hospitalization and an increase in mortality. A cause for anemia is usually found in up to 80% of elderly patients, hence it must be investigated thoroughly and treated appropriately.
Iron deficiency anemia is the predominant form of anemia that occurs in the older population, most commonly as a result of gastrointestinal losses. When it develops acutely, it can become life-threatening if not identified and treated in a timely manner. Patients with anemia must be examined thoroughly for signs of volume depletion and hemodynamic instability. Nonspecific symptoms such as shortness of breath, fatigue, dizziness, confusion and lethargy are often attributed to co-morbidities in an older person or simply aging, resulting in a delay in diagnosis or misdiagnosis. Management decisions regarding anemia should involve functional assessment of the elderly subject. Immediate arrangements for transfusion must be made if the elderly patient is symptomatic irrespective of the hemoglobin level. Evaluation and treatment of iron deficiency anemia can also be performed in the out-patient setting, with potential cost savings and reduced morbidity, length of hospital stay, rehabilitation and premature nursing home placement. Our case series as well as a number of clinical trials have demonstrated that appropriate treatment of anemia can result in substantial improvements in functional capacity, productivity and quality of life of the elderly.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This research was supported by the Claude D. Pepper Older American Independence Center grant (1P30AG28718-01A2). We are grateful to Stephen Foster and Kelly Pollnow for their expert assistance with manuscript preparation.


